Introduction
Semen quality, including ejaculate volume, sperm concentration, and percentages of sperm mortality and abnormality, determines the economic value of boars.1 Polyunsaturated fatty acids (PUFAs), mainly ω-3 and ω-6 acids, are abundant in the plasma membrane of boar spermatozoa2 and provide the sperm plasma membrane, and the fluidity that it needs to participate in the events of membrane fusion associated with fertilization.3 The intake of different types and sources of PUFAs in males can affect the fatty acid composition and quality of semen, hormone levels, oxidative stress and physiological function of the testicle.4,5 Nevertheless, there are inconsistent results for supplementing boar diets with ω-3 and ω-6 as a strategy to improve the quality of seminal doses. Dietary ω-3 and ω-6 supplementation increased membrane-intact spermatozoa6) and contributed to greater progressive sperm motility and antioxidant capacity.1 Other studies suggested that supplementing the diet with oil rich in ω-3 and ω-6 modified the fatty acid composition of the sperm plasma membrane, increased the number of sperm cells in boar ejaculate, decreased the percentage of spermatozoa with abnormal morphologies, and altered some characteristics of their sexual behaviour.7,8
Conjugated linoleic acids (CLAs) are PUFAs that are positional and geometric isomers of linoleic acid (C18:2), which are related to various health-giving properties for animals. In synthetic CLA preparations, the most biologically active and abundant isomers are cis-9, trans-11,9 and trans-10, and cis-12.10 In humans, CLAs possess anticarcinogenic,9 antiatherogenic,11 and antidiabetogenic activities12 and regulate energy metabolism and immune responses.13 In pigs, CLA has demonstrated antioxidant activity14 and may be responsible for changes in whole-body fat deposition.15 The addition of CLA to pig diets modified the type and concentration of other fatty acids and increased the concentration of CLA in the meat.16,17
Currently, the effects of CLA on the reproduction of domestic animals are poorly known, and research has been performed mainly on females.18,19 Therefore, it is not known whether supplementation of the diet with CLA affects semen composition and quality in boars. To clarify whether dietary CLA affects the semen quality of boars, we performed an experiment to evaluate the effect of CLA addition to the diet on the semen characteristics of boars, including the fatty acid profile of sperm, as well as its effects on the carcass (backfat thickness and longissimus muscle area and testicles area).
Materials and methods
Experimental procedures were performed in accordance with the recommendations of the International Guiding Principles for Biomedical Research Involving Animals20 and complied with Mexican law (NOM-062-Z00-1999) for the use of animals in experimentation.21 This research was performed at the Experimental Farm of the Colegio de Postgraduados, Montecillo Campus, located in Texcoco, Mexico State. During the experiment, animals were exposed to the ambient temperatures of October 2008 to January 2009, when the minimum and maximum temperatures were 13.5 and 26.3 °C, respectively
Animals and treatments
Ten sexually active, terminal line boars (Yorkshire × Landrace × Pietrain), 232 ±12.3 kg body weight and 23 months old, were randomly assigned to one of two treatments. After a four-week adaptation, boars were randomly assigned to one of two experimental treatments, consisting of two levels of CLA (0 and 1 %; LutaCLA ® 60, BASF Mexicana S.A. de C.V., Altamira, Tamaulipas, Mexico) mixed in standard diets for boars (Table 1). The diet was formulated based on sorghum-soybean meal to reach the recommended levels of the National Research Council.22
Table 1 Composition of experimental diets
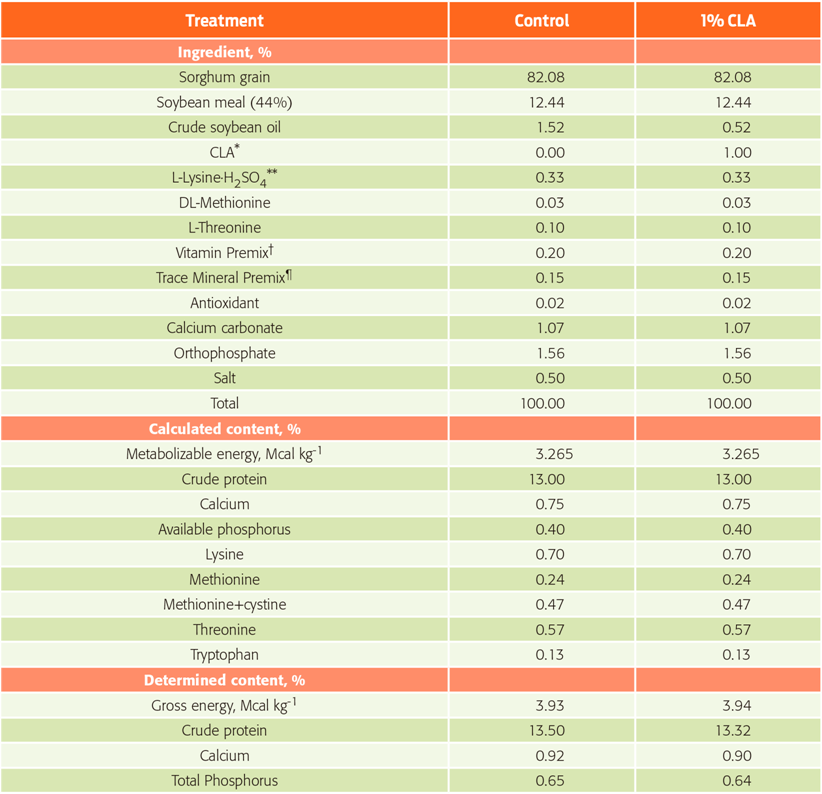
*Conjugated linoleic acid (LutaCLA® 60; BASF Mexicana S.A. de C.V.) contains: cis-9, trans-11 isomers, 30%; trans-10, cis-12 isomers, 30%; other isomers, ≤1%; oleic acid, 22%; palmytic acid, 6%; stearic acid, 4%; linoleic acid, 2%; methanol, ≤100 ppm; heavy metals. **BioLys contains: CP, 75%; lysine, 50.70%. †Each kg of feed supplied: vit. A, 15 000 UI; vit. D3, 2 500 UI; vit. E, 37.5 UI; vit. K, 2.5 mg, thiamine, 2.25 mg; riboflavin, 6.25 mg; niacin, 50 mg; pyridoxine, 2.5 mg; cyanocobalamin, 0.0375 mg; biotine, 0.13 mg; choline, 563 mg; pantothenic acid, 20 mg; folic acid, 1.25 mg. ¶Each kg of feed supplied: Fe, 150 mg; Zn, 150 mg; Mn, 150 mg; Cu, 10 mg; Se, 0.15 mg; I, 0.9 mg; Cr, 0.2 mg.
Data were collected in five replicates per treatment for 12 weeks. However, only data for weeks 0, 4, 8 and 12 were considered for analysis (two treatments × 5 boars × three observations by boar = 30). Boars were individually housed in pens of 4.80 m2 with a concrete floor and equipped with nipple drinkers. The animals had free access to water, and the food was restricted, with 2.8 kg d-1 offered on the floor of the pen.
Semen collection
During the experiment, boars were induced to ejaculate twice per week. Semen was collected using the gloved-hand technique.23 After each boar ejaculated, the total volume was recorded, and the gel fraction was weighed. The sperm-rich fraction was separated from the gel fraction using filter bags for boar semen collection (U.S. Bag™ System, Minitube, Mexico). Semen was maintained at 37 °C in a water bath for evaluation.
Evaluation of semen
The sperm count per millilitre (mL) was determined using a Neubauer chamber. To count the sperm, semen was diluted at a ratio of 1:200 with a solution of sodium citrate 3 % formalin (3 mL formalin in 1,000 mL of double distilled water). Spermatozoa were counted in 5 of the 25 frames of the Neubauer chamber.24 Sperm motility was evaluated by placing a drop of semen on a slide prewarmed to 37 °C, which was observed under an optical microscope at 400x. Total sperm motility was determined as the percentage of sperm that showed any signs of motility or movement in two areas of measurement by counting 100 cells per area.25 The pH of the semen was measured on a sample of 20 mL of semen, immediately after ejaculation, using a portable potentiometer (Orion, Model: 265, Orion Research, Inc. Germany).
Sperm viability and morphology
Assessment of sperm viability was performed using semen smears stained with eosin-nigrosin.26 Sperm morphology was evaluated using sperm smears stained with Bengal rose. Smears stained with eosin-nigrosin and Bengal rose were evaluated by counting 200 sperm per smear with an optical microscope at 400x. Viable sperm were defined as those that did not stain. Sperm morphology was evaluated by quantifying the proportion of sperm with an abnormal head, tail, middle part, or the presence of cytoplasmic droplets.27 Individual sperm abnormalities were added to obtain a single value of sperm cell abnormalities per ejaculate.
Carcass and testicular characteristics
From the beginning of the experiment and at weeks 4, 8 and 12 of the experimental period, backfat (BF) thickness and longissimus muscle area (LMA) were measured at the tenth rib level using a real-time ultrasound Sonovet 600 with a 3.5 MHz transducer (Medison, Inc., Cypress, California, USA). Additionally, the cross-sectional area (cm2) in the right (RTA) and left (LTA) testicles, at approximately the middle of the testis, was measured using the same ultrasound.
Laboratory analysis
Samples of sperm cells at weeks 0, 4, 8 and 12 for each boar were collected for fatty acid analysis. The semen was centrifuged at 1,500g for 20 min at 4 °C to separate sperm cells from seminal plasma. After separation, sperm cells were resuspended with 50 mL of a solution containing BTS (Beltsville Thawing Solution; Minutube, Mexico) and then recentrifuged. The washed sperm cells were stored at -20 °C for fatty acid analysis.
Determination of total lipids in sperm cells and experimental diets was performed following the 923.07 method of AOAC 2000.28 Saponification of the total lipids was performed with 2 % methanolic sodium hydroxide, using myristoleic acid (Sigma-Aldrich Co., USA) as internal standard for gas chromatographic analysis. Trans-esterification to methyl esters of the lipids in sperm cells and diets was performed according to the 969.33 method of the AOAC 2000,28 using boron trifluoride-methanol (Sigma-Aldrich Co., USA). Fatty acids were identified and quantified by gas chromatography on a Varian 3400 CX chromatograph, equipped with an autosampler and a flame ionization detector (Varian Inc., CA, USA) using N2 as the carrier gas at a×m flow of 30 mL min-1. Temperatures used for chromatography were as follows: column, 230 °C; injector, 150 °C; and detector, 300 °C for fatty acids. Fatty acid methyl esters were identified by retention times relative to a standard mix (FAME mix C4-C24. Sigma Aldrich Co., MO, USA). CLA isomers were identified using a methyl ester standard that contained an acid mix of cis-9, trans-11; trans-9, cis-11; trans-10, cis-12; cis-10, and trans-12. (Cat. No. 05632, Sigma Aldrich Co., MO, USA). The fatty acid concentration of the samples was calculated using the area under the curve relative to the known standard.
The experimental diets were analysed in the laboratory to determine the concentration of crude protein using the Kjeldahl method.29 Gross energy was measured in an adiabatic calorimeter bomb (Oxygen Bomb Calorimeter, Parr Instruments Co. Illinois, USA), following the methodology of Tejada, 30 and calcium and total phosphorus were measured by atomic absorption spectrophotometry (Varian Spectrophotometer SpectrAA 10 plus, Varian, Australia) following the methodologies of Fick et al.31
Statistical Analysis
Before analyses, the Shapiro-Wilk and Levene’s test were used to corroborate the normal distribution and variance homogeneity of the variables data. The experimental design was a completely randomized design with treatment, week and interaction (treatment × week) as fixed effects, and the boar as random effect. Differences between treatments in the fatty acid profile of sperm cells, seminal characteristics, carcass and testicular characteristics were analysed using the mixed effect tests of SAS,32 in which initial values of the fatty acid profiles of sperm cells, seminal, carcass and testis characteristics were used as covariates for the statistical analysis of its corresponding variable for each boar. To determine the effect of CLA intake, time and the interaction between CLA and time, orthogonal linear and quadratic polynomials were used.33 Statistical significance of the results was set at P ≤ 0.05.
Results and discussion
In this experiment, it was assumed that a period of 84 days of experimental diet intake was sufficient to observe and confirm the effects of the intake of CLA on sperm quality and to maximize the incorporation of long-chain fatty acids into the plasma membranes of sperm. This was supported by the duration of spermatogenesis, which lasts between 39 and 40 days and because spermatozoa spends 9 to 12 days in passing through the epididymis of the boar.34 Furthermore, the effects of supplementing the boar diet were observed after 56 days.8
Fatty acid profile of oils and experimental diets
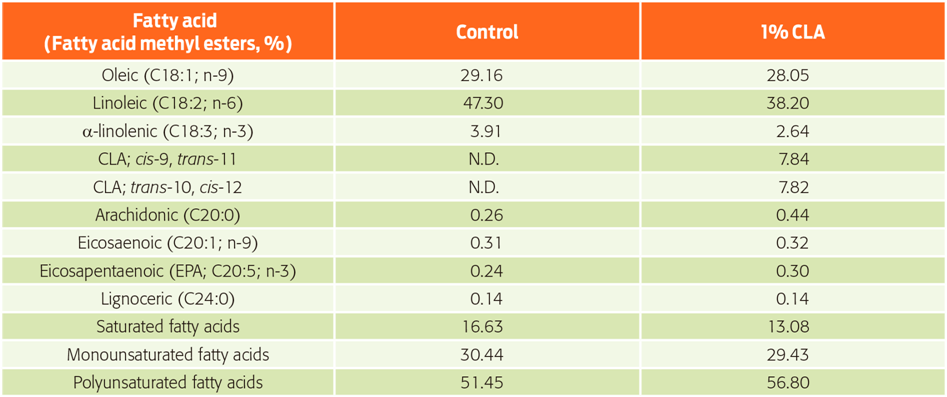
Analysis of the fatty acid profile of the experimental diets (Table 2) shows that diets with 1 % CLA had 7.84 % and 7.82 % of the isomers cis-9, trans-11 and trans-10, cis-12, respectively. These isomers were not detected in the control diet.
Seminal characteristics (Table 3)
Table 3 Seminal characteristics of boars fed diets supplemented with CLA (n = 30; error degrees of freedoms = 24)
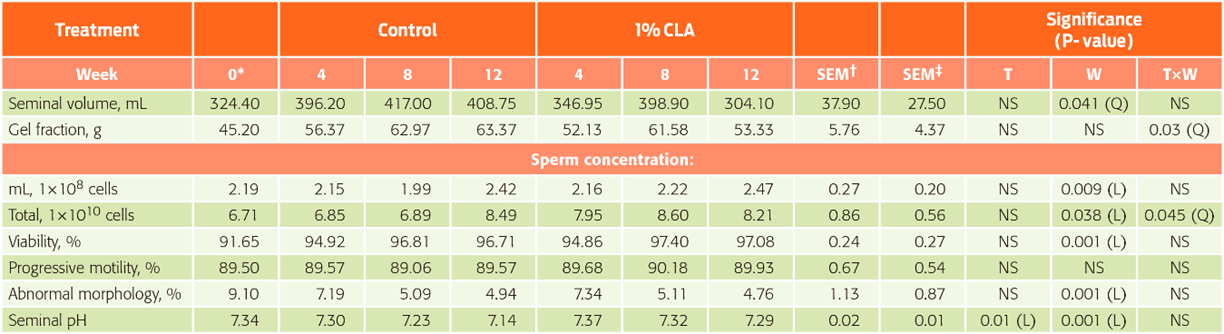
*Initial values used as covariates. † Standard error of the mean for treatments; ‡ Standard error of the mean for weeks. T = treatment; W = week; T×W = interaction between treatment and week.
Effects: L = linear; Q = quadratic; NS = non significant.
Only the semen pH was affected by the CLA diet (linear effect, P = 0.010). Over time, a decrease in seminal pH was observed in both treatments (linear effect, P = 0.001). The semen pH of boars fed the control diet showed a greater decrease (linear effect, P = 0.001). The semen volume increased from week 4 to 8, showing a reduction at week 12 of the experiment (quadratic effect, P = 0.044). Changes in semen volume affected the sperm count per mL, with a greater concentration of sperm per mL when the volume of semen decreased (quadratic effect, P = 0.044). Furthermore, the overall sperm concentration improved during the experimental period (linear effect, P = 0.009), with a greater increase in the total concentration of sperm in pigs fed the control diet (quadratic effect, P = 0.038). The sperm viability improved (linear effect, P = 0.001), whereas the number of cells with an abnormal morphology decreased (linear effect, P = 0.001).
No changes in seminal characteristics were observed due to CLA intake, similar to other studies in boars in which PUFAs supplementation had no effect on semen quality.35,36 However, other experiments supplementing boar diets with PUFAs ω-3 and ω-6 improved the quality of seminal characteristics, modifying the fatty acid composition of the sperm.1,6,7) Although seminal characteristics were not affected by CLA intake during the experimental period, the increase in sperm concentration and viability and the decrease in abnormal morphology may be related to seasonal changes in temperature. Previous studies performed in colder months suggested that during the cooler months of the year, sperm count and semen production improved,37 and sperm abnormalities were fewer in pigs housed in cool temperatures.38 In our study, seminal pH was the only variable affected by diet and time. This indicates that CLA intake can affect the secretions of the prostate, seminal vesicles and Cowper’s gland, suggesting that CLA facilitate the good health of these glands.37
Fatty acid profile in sperm cells
The fatty acid profiles of sperm are presented in Table 4. The total concentration of saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs) and PUFAs was not affected by adding CLA to the diet (P > 0.05). Only the concentration of PUFAs was affected by time (quadratic effect, P = 0.008). During the experimental period, the concentration of eicosatrienoic (P = 0.001), arachidonic (P = 0.001), and docosahexaenoic (P = 0.047) acids, as well as the total concentration of n-3 fatty acids (P = 0.018), had a quadratic relationship with time, showing a decrease of these fatty acids at week 8, and either an increase in concentration at week 12 or similar to the values at the fourth week, whereas the concentration of total n-6 fatty acids increased over time (P = 0.050). Furthermore, the relationship between the n-6 and n-3 fatty acids (quadratic effect, P = 0.043) and the relationship between docosahexaenoic and docosapentaenoic acids (linear effect, P = 0.038) showed the same behaviour as the total n-6 fatty acids.
Table 4 Fatty acid profile of boar spermatozoa (g per 100 g of total lipids) (n = 30; error degrees of freedom =24)
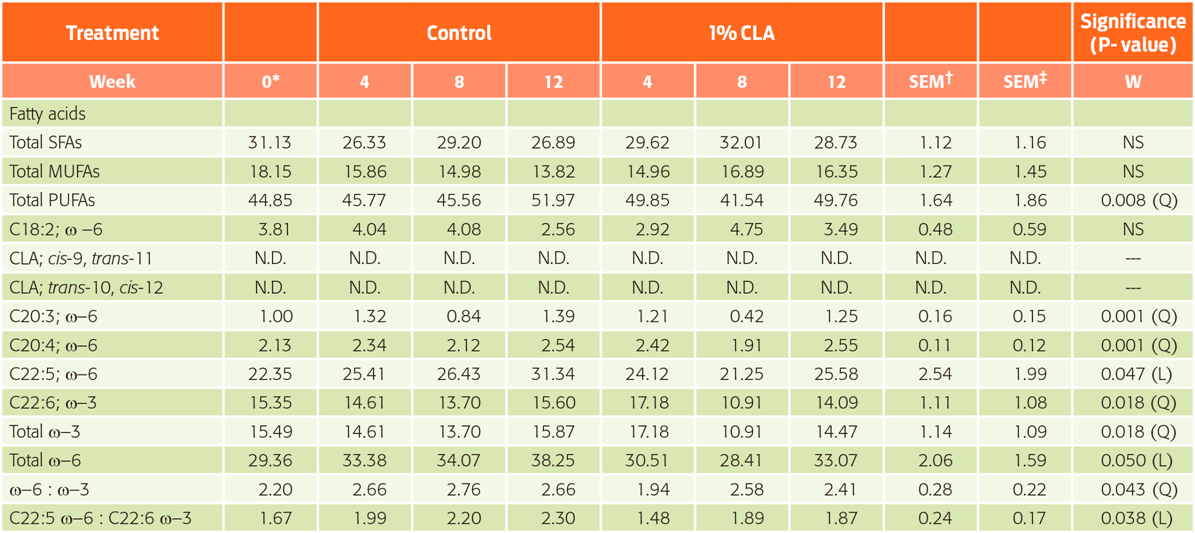
*Initial values used as covariates. † Standard error of the mean for treatments; ‡ Standard error of the mean for weeks. W = week; SFAs = saturated fatty acids; MUFAs = monounsaturated fatty acids;
PUFAs = polyunsaturated fatty acids; N.D. = non-detectable.
Effects: L = linear; Q = quadratic; NS = non-significant.
No changes in the fatty acid profile of boar semen with supplementation of CLA in this study were observed. In most animal species, the fatty acid profile in sperm and semen reflects fatty acid intake.5 Specifically, pigs are effective in transferring fatty acids from the diet to the germ cells.6,7
Previous studies found that the fatty acid composition of sperm was modified with PUFAs supplementation6,8,36, suggesting that PUFAs supplementation changes the lipid composition of the semen of boars. However, our results were contrary to these findings, and these differences may be due to the breeds used in the experiments. Yeste et al. noted that breed differences in boars fed with omega-3 could explain controversial reports in the literature,39 and may be related to differences in the composition of plasma membranes among breeds reported by other authors. They did not find negative effects in the three breeds evaluated (Duroc, Large-White and Pietrain) and found only positive effects in sperm quality in Large-White and Pietrain boars. The authors concluded that omega-3 fatty acids may improve sperm quality; however, this depends on the boar breed. These controversial results may be due to variations in the quantities and sources of oil and the duration of supplementation.
Carcass and testicular characteristics
For carcass and testicular characteristics (Table 5), the intake of CLA linearly increased the backfat thickness (P = 0.008). No differences were found between treatments during the course of the experiment (linear effect, P = 0.045), but backfat accumulation occurred with lower intensity in pigs fed the control diet. Furthermore, the LMA increased (linear effect, P = 0.011) only as an effect of time. Regarding the transverse testicular area, although this characteristic was not affected by CLA intake (P > 0.05), there was an increase in cross-sectional area of both testicles during the experimental period. The decrease in seminal volume and number of total cells in the CLA diet animals, considering that these animals showed an increase in testicle area, could be due to CLA addition increasing lipid peroxidation. This is due to the observation that higher linoleic acid can cause the cascade reaction of lipid peroxidation and DNA damage of spermatozoa.40
Table 5 Carcass and testicular characteristics of boars fed CLA supplemented diets (n = 30; error degrees of freedom = 24)
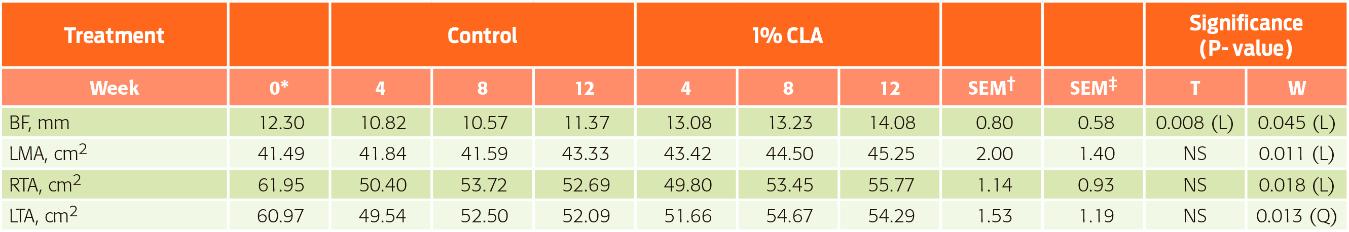
*Initial values used as covariates. †Standard error of the mean for treatments; ‡Standard error of the mean for weeks. T = treatment; W = week; T×W = interaction between treatment and week;
BF = backfat thickness; LMA = longissimus muscle area; RTA = right testicle area; LTA = left testicle area.
Effects: L = linear; Q = quadratic; NS = non-significan
The greatest accumulation of backfat observed in pigs fed CLA diets is inconsistent with the findings in growing-finishing animals, in which inclusion of 0.5 % CLA in diets produced no change in backfat thickness.16,41 By contrast, the addition of 4 % CLA tended to reduce backfat in gilts.42 Due to high levels of dietary PUFAs, they may promote lipid peroxidation and limit the viability and motility of spermatozoa.43 There is evidence that CLA may inhibit the proliferation and/or differentiation of adipose cells and the synthesis and accumulation of triglycerides in adipocytes, decreasing body fat deposition.44
With respect to LMA, the results are consistent with those of Mirand et al,45 which indicate that the intake of CLA in adult animals does not change the body composition. Moreover, the increased cross-sectional area of the testes during the experimental period may be due to the increased production of semen.











 nueva página del texto (beta)
nueva página del texto (beta)