Introduction
Rabies is a major viral zoonosis that affects mammals, including humans, and is globally distributed with a few notable exceptions. The rabies virus, transmitted in the saliva of sick animals by direct contact through deep bites or scratches, may be fatal once the infected individual develops clinical signs.1 Rabies is caused by the Rabies lyssavirus virus (RABV), one of the 11 identified virus species of the genus Lyssavirus (family Rhabdoviridae). It is widely distributed throughout the world, and is the only one reported in America.2 To date, nine antigenic variants of this genotype have been reported in Mexico.3,4 Rabies is maintained in two epidemiological cycles, urban and sylvatic. In the urban rabies cycle, dogs are the main reservoir host, whereas wild mammals (i.e., carnivores and bats) are reservoirs of the sylvatic cycle.5
Globally, approximately 55 000 people die annually from rabies, and most victims are children in underserved rural areas of Africa and Asia.6 In temperate environments, the primary reservoirs maintaining rabies are carnivores and insectivorous bats.7 However in tropical environments, the vampire bat (Desmodus rotundus) is the primary reservoir responsible for its transmission.4,8 In the last decade transmission of rabies by bats has become a significant public health threat in some parts of the world.6,9,10 In Mexico, the transmission of rabies between carnivore species and bats does not appear to be a frequent occurrence,2 but this assessment may be biased towards vampire bats because of the economic impact to the livestock industry.11,12 Annual losses to rabies in the livestock industry are estimated at more than $23,000,000.00 US dollars (NOM-067-ZOO-2007)11 and effective management requires epidemiological surveillance and weekly notifications to the National Epidemiological Surveillance System (SIVE) and the National Service of Agro-Alimentary Health, Safety and Quality (Senasica). In 2012, the national campaign against bovine paralytic rabies assisted 1 112 bovine farms that reported bat attacks, with 360 confirmed rabies cases in cattle.13
The common vampire bat, a habitat generalist species with wide ecological plasticity that inhabits the tropical and subtropical regions in Mexico and south to central Chile and northern Argentina.14,15 In Mexico, D. rotundus has been recorded in 24 states, from central Sonora in the western part of the country eastward to the coastal plains of the Gulf of Mexico in northern Tamaulipas, and from the Pacific coast to the Yucatán Peninsula and Chiapas.12,13 It is commonly found in environments with warm temperatures (between 20 to 27 ° C) and is best suited where there are little fluctuations (24 to 25 °C) in their roosts; D. rotundus can tolerate a temperature range from 35 to 37 °C.(16,17) While the optimum range of relative humidity in their shelters is 70 to 100 %, it should not drop below 45 % for survival.15
The geographic range of D. rotundus in Mexico does not extend outside the 10 °C minimum temperature isotherm or above an altitude of 2 300 masl (meters above sea level.)14,16 Some studies suggest that if climatic conditions change, individuals are likely to relocate to new areas and roosts.18,19 Due to its ecological plasticity, the vampire bat can easily adapt to new landscapes modified by humans where loss of native plant cover, increased forage and greater abundance of domestic livestock have created suitable conditions for their breeding and feeding.4,8 In Mexico, it is expected that the combination of changes in land use and changes in global and regional climates will produce species distributional shifts, including reservoirs and vectors for infectious diseases, modifying the patterns of infectious disease occurrence. Thus, modelling species distribution in changing scenarios is becoming a major tool for conservation biology and for public health.
The ability to predict species distribution based on presence records may allow predicting the presence and absence of species in areas not previously sampled.20,21 If such predictions prove to be robust, it is possible to predict the response of species to the effects of global climate change. The ecological niche models (ENMs) have been used to predict the geographic range of species based on the relationship between environmental variables (e.g., biotic and abiotic variables) and the presence data of species.22 ENMs are founded on the classical concept of “niche” in ecology.23 This concept is based on two previous niche concepts; the first approach is based on environmental conditions such as temperature, precipitation, soil, etc., for which biological interactions such as competition are not relevant.24
This approach is widely used to understand coarse-scale ecological and geographic properties of species,21 instead the second approach considers that biological interactions such as competition, determine the geographic distribution of species.25
The latter approach is used to understand the fine-scale ecological and biological relationships between species.21
The objective of this study was to understand the current geographic distribution of D. rotundus under climate change scenarios in Mexico. We developed niche models to identify the current and future distribution patterns of D. rotundus, and compilated a database of bovine paralytic rabies cases to identify the areas of highest risk for bovine rabies, and to facilitate the development of preventive strategies in the country.
Materials and methods
Study area
The study area comprises the entire territory of Mexico, between 14° N and 32° N, 86° W and 118° W. Mexico shares land borders with the US (to the north) and Guatemala and Belize (to the south). The total area of Mexico is approximately 1 900 000 km2 and is characterized by a great diversity of landscapes and climates. Approximately 85 % of the country (except the Yucatán Peninsula and the coastal plains of the east and northwest) is formed by mountain ranges, plateaus and numerous valleys.
Desmodus rotundus occurrence data
To generate the present potential distribution map for D. rotundus, the study area was delimited using the known distribution of the species in Mexico.
We collected D. rotundus occurrence data from the National Mammal Collection of the Institute of Biology and the Museum of Natural History of the National University of Mexico (UNAM), the Mexican Commission for the Knowledge and Use of Biodiversity (Conabio), and the Global Biodiversity Information Facility.26 Data collected for each record included locality (geographic coordinates in decimal degrees), height above sea level and source of information. We used only records from 1980 to 2013, avoiding old and imprecise data.
Bovine paralytic rabies and population data
We used animal health weekly reports from the National Agro-Alimentary Health, Safety and Quality Service (Senasica),27 to develop a database of cases of bovine paralytic rabies from 2007 to 2015. We also used information about the national pastoral range-livestock population from the Agricultural, Livestock and Forestry Census 2007.28 This data will be used to identify areas with more cattle and potential riesk areas. The information in both data sources was provided at the municipal level.
Environmental data
For this study, a total of 20 environmental variables were considered including 19 bioclimatic variables from temperature that reflect seasonal and annual trends of temperature and precipitation (Table 1). These variables were obtained from the WorldClim database 29 for the 1950-2000 period. We used data for elevation above sea level, obtained from the Digital Elevation Model generated by the National Institute of Statistics, Geography and Informatics30; and used ArcGIS 10.231 to perform spatial analyses at a resolution of 30 arc-seconds (~ 1km2).
Table 1 Contributions of environmental variables used in the model of Desmodus rotundus in Mexico.
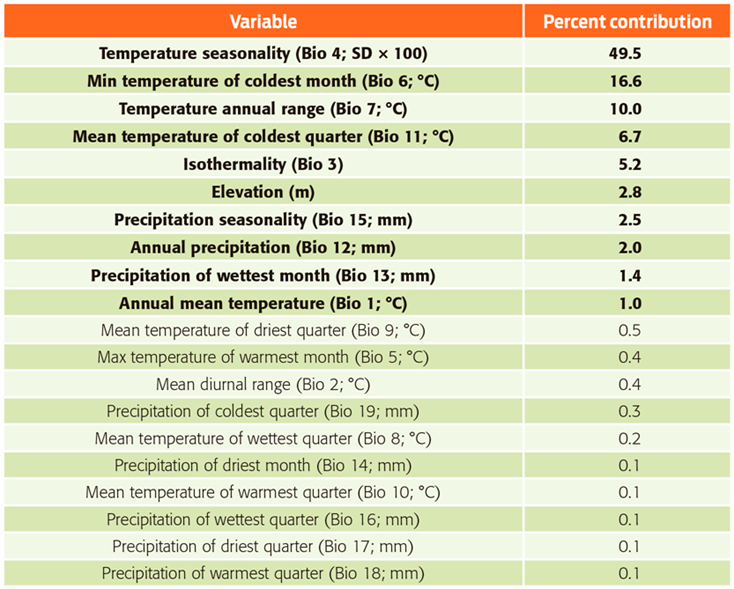
Note: Bold font indicates variables that contribute ≥ 1 % to the model.
Source of data: WorldClim.29
We used two Global Circulation Models (GCMs) to determine the likely distribution patterns of D. rotundus in Mexico for the decades of 2050 (average 2041-2060) and 2070 (average 2061-2080) at a spatial resolution of 30 seconds: Geophysical Fluid Dynamics Laboratory Model, version 3 (GFDL-CM3) and Hadley Centre Global Environment Model-ES version 2 (HadGEM2).29 These climate change predictive models were proposed in the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC) and the Coupled Model Intercomparison Project, Phase 5.32 Both GCMs considered two greenhouse gas concentration trajectories or Representative Concentration Pathways (RCP); 4.5 and 8.5. The former assumes annual emissions will decline near the year 2050 and a conservative increase in population growth, while the latter assumes CO2 and methane emissions will continue to rise throughout the twenty-first century and a rapid increase in the human population.32
Ecological niche model
We used the correlative maximum entropy based model or MaxEnt program 33,34 to assess the potential distribution of D. rotundus in Mexico. MaxEnt is a robust algorithm that provides informative results and requires presence-only data.20,35-37 MaxEnt is a correlative model based on an adjustment Bayesian procedure under the principle of maximum entropy that is used to estimate probability distributions (i.e., more uniform distributions) based on records of the presence of a species. It is subject to restrictions given by the environmental information or other variables such as topography.33,34 To build the model, we divided the total number of records into two sets, a calibration data set (random selection of 75 % of the data as training data) and a set of test data (25 %) in 100 replicates in 500 iterations with different random partitions (bootstrap method). To test the performance of the model and its discriminatory capacity, we used the AUCtest (area under the ROC curve based on the testing data), where values close to one show a good prediction and values close to 0.5 indicate that the model behaves randomly.33,38 It is important to note that although some studies have identified the limitations of the AUCtest as a performance measure of models,39,40 this test is often used as a single threshold-independent measure for model performance and is a highly effective measure of the performance of ordinal score models.36,41 The statistical significance of the model was evaluated using a binomial test which determines whethe the models are better than randomly obtained models.33,38 Likewise, a Jackknife test was conducted to measure and determine which of these implemented variables were the most important for the presence of the species.33 Subsequently, we generated models of current and future distribution based on the probability threshold that minimizes the error of omission (≤ 10 % training and validation) and the fraction of the predicted area to avoid overprediction.20,38,42 We used a multivariate environmental similarity surface (MESS) to assess the level of similarity of the environmental conditions of the predicted areas between the two GCMs employed.36 The results allowed us to distinguish areas of model extrapolation from those with similar environmental conditions at any site in the world.
An additional component in developing niche models is the mobility area (M). This area is frequently much larger than the actual distribution of the species in question.43 Therefore, the predictions represent hypotheses about conditions that are similar to those where the species has been observed. These conditions are likely to be among the existing fundamental niche and the niche occupied by the species.20 Because Desmodus rotundus has a wide dispersal capacity and information about the natural history is available, we considered the entire country of Mexico as our M in the models. Currently the species is not present in the northern states of Mexico such as Baja California, Sonora, Chihuahua and Coahuila.13,15 However, these areas represent a potential opportunity to be colonized, if they present suitable environmental conditions in the future.
Areas at risk of bovine paralytic rabies
Predicted areas were identified as high risk due to probability estimates of the potential occurrence of D. rotundus in the study area. We superimposed maps of current and future D. rotundus distributions with database records of bovine paralytic rabies from Senasica27 to identify municipalities with potential risk of disease. We then input the layer of estimated number of free-ranging cattle from the Agricultural, Livestock and Forestry Census 2007,28 in each area to assess the potential extent of cattle susceptibility to the disease.
Results and discussion
Bovine paralytic rabies distribution
Bovine paralytic rabies remains a threat for livestock production in Latin America and Mexico where the vampire bat, Desmodus rotundus, is the main vector.4,11 From 2007 to 2015, 119 389 cases of bovine paralytic rabies were reported, of which 1 872 were confirmed, distributed in 477 municipalities in 25 Mexican states. States with the highest number of confirmed cases were Veracruz (342 confirmed cases, 25 923 reports), San Luis Potosi (211 confirmed cases, 2 624 reports), Yucatán (190 confirmed cases, 15 783 reports), Tabasco (181 confirmed cases, 16 132 reports), and Chiapas (127 confirmed cases, 13 181 reports). States with the lowest number of reported cases were the State of Mexico, Guanajuato and Baja California Sur. Municipalities with the greatest number of positive cases of bovine paralytic rabies in the same period were: Tierra Blanca (75) and Papantla (61) in Veracruz, Huimanguillo in Tabasco, Mapastepec (60) in Chiapas, and Candelaria (60) in Campeche.
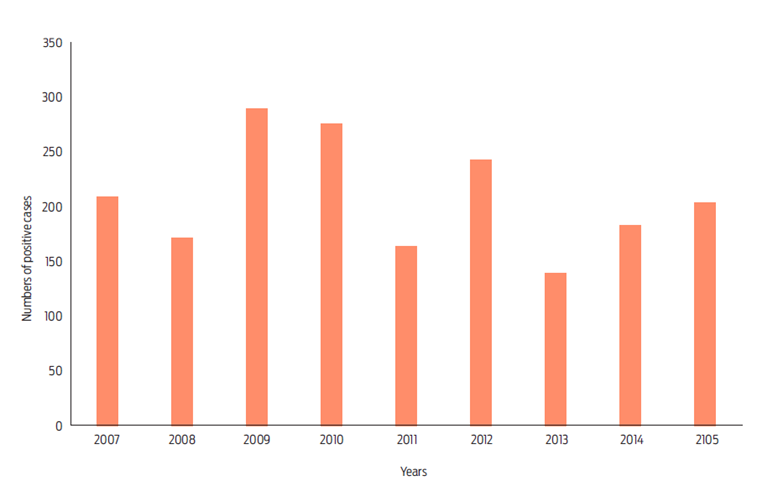
The annual number of confirmed cases fluctuated between 138 and 288, recorded three maxima: 288 cases in 2009, 275 in 2010 and, 243 in 2012 (Figure 1). When comparing seasons, we observed that most cases were reported during the dry season; a total of 1064 cases were reported between January and May, with two peaks in March and May (200 and 234 cases, respectively; Figure 2). Note that in this study we used correlative maxima entropy models to develop maps of the current distribution of suitable Desmodus rotundus habitats in Mexico. We then used climate change predictions to determine how changes in bioclimatic factors influence potential distribution of D. rotundus, the principal vector of rabies virus within pastoral range-livestock production systems, to identify potential bovine paralytic rabies risk areas.12
Current distribution of Desmodus rotundus
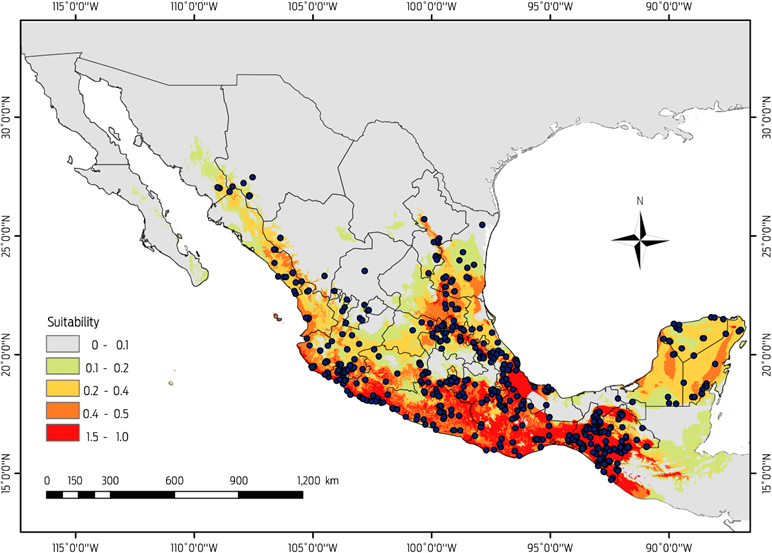
The current potential distribution of D. rotundus we generated from the 649 confirmed records for the period 1980-2013, performed well and had good adjustment (AUCtest value of 0.95 ± 0.029) and a low rate of omission (P < 0.0002). The most important variables associated with the presence of D. rotundus (Table 1) were temperature seasonality (49.5 %), followed by minimum temperature of coldest month (16.6 %) and annual temperature range (10 %). The areas identified with a greater likelihood of the presence of D. rotundus maintain a temperature difference of less than 4 °C between the colder and warmer quarters, and both variables had low values, indicating low variation in temperature throughout the year.
Model prediction results for the current scenario indicate habitat suitability for Desmodus rotundus covers an area of 834 965 km2 (Figure 3), and the areas with the most appropriate conditions (probability > 0.49) are located along the coastal areas: The Pacific coast, from southern Sonora to the southwestern corner of the country, and the Gulf of Mexico coast, from central Tamaulipas to the Isthmus of Tehuantepec, Chiapas and the Yucatán Peninsula (Figure 3). Areas with a high probability of suitable conditions were primarily in the states of San Luis Potosi and Veracruz and in the southeastern region of the country. There were areas in the northern regions of the country, such as the states of Tamaulipas, Sonora and Sinaloa, in which D. rotundus are present in tropical and subtropical environments with warm temperatures that do not fluctuate more than 2 °C annually. However, the transformation of natural environments to grasslands for the development of the cattle industry, and the micro-climatic changes fostered by these disturbances have favoured the expansion of D. rotundus into other regions.12,15,44
Future distribution of D. rotundus
Predicted risk maps for the 2050 and 2070 climate scenarios show a reduction in potential distribution of Desmodus rotundus as previously identified in the current scenario. The best predictive model of D. rotundus distribution under each of the two GCMs, GFDL-CM3 and HadGEM2, had AUCtest values of 0.97 ± 0.02, indicating a high predictive power for both models. Under a conservative scenario of RCP 4.5, the distribution models under both GCMs show a marked reduction of total area compared to the current values: a reduction of 20 % (170 317 km2) for the decade of 2050 and 28 % (233 989 km2) for the decade of 2070 (Figures 4, 5). A similar pattern was observed when the more development-intensive RCP 8.5 scenario was used: a reduction of 27 % (229 486 km2) for the decade of 2050 and 34 % (283 471 km2) for the decade of 2070 (Figures 4, 5). Comparison of the species distribution models revealed that with respect to the current geography, GFDL-CM3 GCM predicted reductions of 15 % and 33 % for both periods of time, whereas HadGEM2 predicted a reduction of 9 % and 12 % for 2050 and 2070, respectively.
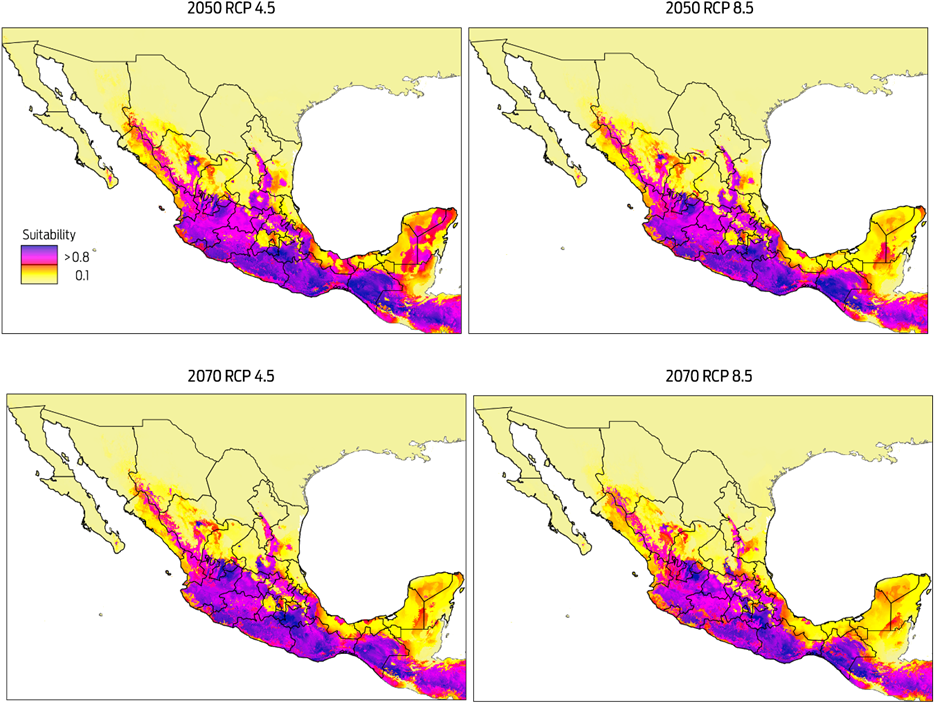
Figure 4 Modelled suitability for future distribution of Desmodus rotundus according to Global Climate Model GFDL-CM3 for two time periods (2050 and 2070), and two Representative Concentration Pathways (RCP 4.5 and 8.5). Left-hand column shows suitability values, with blue indicating more suitable conditions.
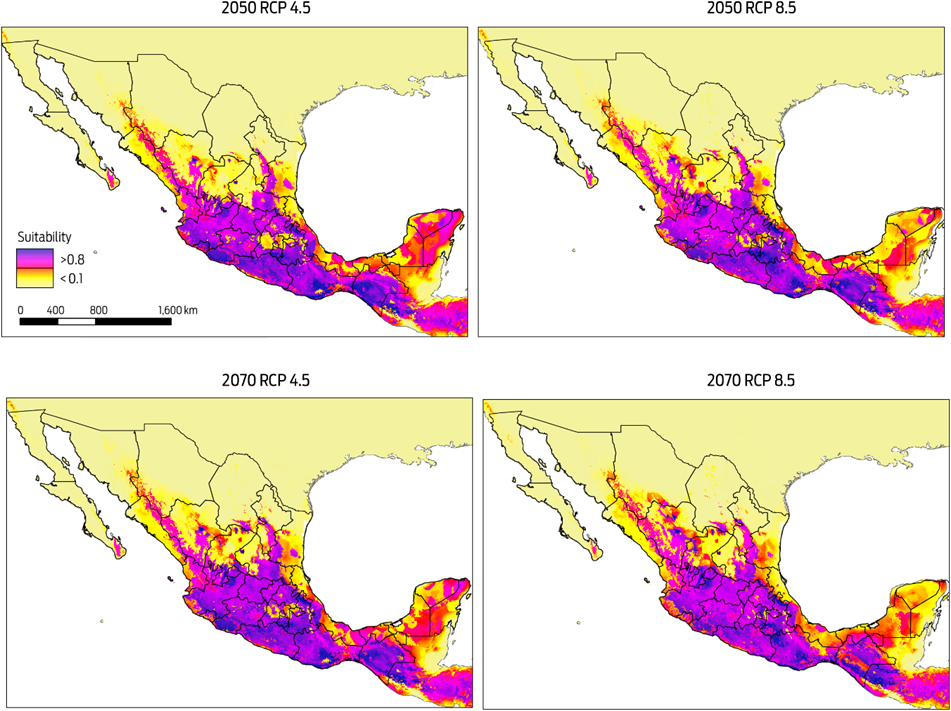
Figure 5 Modelled suitability for future distribution of Desmodus rotundus according to Global Climate Model HadGEM2 for two time periods (2050 and 2070), and two Representative Concentration Pathways (RCP 4.5 and 8.5). Left-hand column shows suitability values, with blue indicating more suitable conditions.
The MESS analysis, which showed broad areas of similar environmental conditions under current and predicted future climatic conditions, also identified suitable areas where D. rotundus is currently absent, including central Mexico, Coahuila, Baja California, western Sonora, and northern Chihuahua. Under all IPCC climate change scenarios analyzed, D. rotundus expanded its range into the northwest of the country, occupying new areas in the states of Sonora, Sinaloa, Zacatecas, Aguascalientes, and Guanajuato, the northeast regions of Mexico in the states of Coahuila, Nuevo León and San Luis Potosi, and the Central Mexican Plateau, principally the states of Mexico, Puebla and Hidalgo.8,12 In contrast, the reduction in distribution occurred primarily in the southeastern portion of the country, notably in the Gulf Coastal Plain in southern Veracruz, Tabasco, southwestern Campeche, and the Yucatán Peninsula (Figures 4, 5).
It is important to note that while there may be some shift in the geographic patterns of the distribution of D. rotundus under future climate change scenarios, these changes are not significant on a nation-wide scale, and the species will continue to be located primarily in tropical and subtropical regions of the country.12,15 However, an increase in the suitable area for other vectors of the rabies disease and other biotic factors driving the dynamics of the disease, such as shelter and food availability and biological corridors, may contribute to an increased risk of the disease.15,44,45
Our results indicate significant changes in the pattern of distribution and incidence of bovine paralytic rabies in Mexico. The highest risk for bovine paralytic rabies occurs in the dry season (April and May). This pattern is consistent with reports of a spike in the number of rabies cases in Brazil, Colombia and Costa Rica during the dry season.46,47 We identified temperature as a principal determinant in the distribution of D. rotundus; it affects the spread of the rabies disease among tropical and semi-tropical environments and, the likelihood of its spread into more temperate areas that may experience increased temperature. We estimate that more than 600 000 head of cattle in Mexico could be exposed to bovine paralytic rabies virus by 2050-2070, according to the current number of free-ranging cattle from the Agricultural, Livestock and Forestry Census 2007.28 Nevertheless, an increase of suitable areas for other vectors of the rabies disease and other biotic factors driving the dynamics of the disease, such as shelter and food availability and biological corridors, may contribute to the increased risk of the disease.8,44,48 This number is likely an underestimation because the cattle population is likely to exceed the values reported by the 2007 census that were used in the model and because of the ongoing and projected conversion of natural ecosystems to grazing land to accommodate livestock production.
Conclusion
Identification of current and future areas that are likely to support vectors of bovine paralytic rabies is a critical economic and social challenge that requires immediate attention. Bioclimatic conditions are a good predictor of the current distribution of one primary vector, Desmodus rotundus, and we used this relationship to show that by 2050-2070, 30 % of the Mexican landscape will provide a suitable habitat for this species because of changing climatic regimes. This areal expansion will occur into northern and central Mexico where D. rotundus populations are currently minimal or absent. The increase in the area of cattle production and D. rotundus habitat overlap is a serious threat to the industry because of the likely increased incidence of bovine paralytic rabies. It is necessary to identify strategies, such as strengthening the National Epidemiological Surveillance System and monitoring programmes, to effectively maximize resources to address this challenge. These programmes will also benefit community health by reducing the probability of human rabies infections spread through vampire bat bites.











 nova página do texto(beta)
nova página do texto(beta)