Introduction
The horn fly, Haematobia irritans (L.), is common and primarily affects cattle on grass pastures in Mexico. The horn fly was introduced from Europe to North America in the late 19th century. Currently, it is widely distributed across the American continent and is considered the major pest of livestock1,2. H. irritans is an obligate bloodsucking ectoparasite, always remains on cattle and feeds 24 to 38 times a day, consuming an average of 1.71 mg of blood per meal.2 The feeding habits of horn flies on cattle cause irritation and discomfort. In an effort to repel the flies, the animals develop defensive behaviours such as tail flicks, leg stomps, skin twitches and head throws. This stress, which varies in intensity based on the quantity of flies, results in reduced grazing time and increased energy spent.3-5 Hence, treating horn fly infestations are of sanitary and economic importance.
It has been estimated that weight loss can be up to 220 g/day and milk production can be between 10 and 20 % of regular production when the infestation is high (> 300 flies/animal). Furthermore, H. irritans is an efficient transmitter of several viral, bacterial, protozoal, and rickettsial diseases and helminth infections.2,6,7 The bites of the horn fly reduce the quality of the hides, and insecticide application is costly. In general, control measures of the horn fly for grazing cattle include applying insecticides of different chemicals through the use of earrings, aspersions, immersion baths and pour-on formulations; however, the resistance to insecticides is present in several livestock regions of South America.8-10 In Mexico, the problem is more severe in tropical regions.11-14
The use of insecticides has a considerable impact on public health due to the contamination of meat and milk with toxic residues and through environmental pollution. An alternative for reducing the intensive use of insecticides is to use non-chemical measures such as biological control. For example, the entomopathogenic fungi is a safe and sustainable alternative with minimum risk for vertebrates, humans and the environment. This fungus infects the insect through contact, invading it through the cuticle and killing it. After the death of the insect, the fungus emerges from the cadaver to produce new conidia.15
Currently, the efficacy of the fungus Metarhizium anisopliae as a biological control of H. irritans has been demonstrated under laboratory conditions in both immature and adult stages,16-19 as well as in adults under controlled infestation conditions.20 However, the efficiency of this entomopathogenic fungus has been scarcely studied under field conditions.21 To advance the development of this biological control, the objective of this study was to determine the effectiveness in reducing the infestation of H irritans on dairy cattle under natural conditions, by applying the strain Ma134 of M. anisopliae by aspersion.
Material and methods
Study site
This work was performed at the livestock unit of El Llano Technological Institute, located in a municipality of the same name in Aguascalientes, Mexico. The place is 2 020 masl, with a semiarid climate, summer rains, and an average temperature of 15.5 °C. In the region, natural infestation of H. irritans is seasonal, being the highest from summer to autumn.22,23
Entomopathogenic fungi
The strain M. anisopliae sensu lato 134 (Ma134) was isolated from soil in the dairy unit at El Llano Technological Institute using the larvae of Galleria mellonella L. (Lepidoptera: Pyralidae) as a reservoir host. Taxonomic identification was based on morphological criteria of the reproductive structures; the strain forms part of the entomopathogenic fungi collection at the same Institution.24 The strain Ma134 was evaluated in Musca domestica and Stomoxys calcitrans adults under in vitro conditions with good results24 and high efficacy with S. calcitrans under field conditions.25 The strain was cultured in the laboratory according to the protocol previously described by Cruz-Vázquez et al. 25 The isolate was cultured on Sabouraud’s Dextrose Agar enriched with 1 % yeast extract26 containing 500 ppm chloramphenicol and incubated at 25 ± 1 ºC for 21 days in a 12:12 hr light/dark regime. Conidia were harvested by scraping, suspended in sterile distilled water containing 0.1 % (v:v) Tween 80, and homogenized on a vortex mixer. Spore viability, which exceeded 98 %, was determined by seeding 100 µl of conidial suspension on Sabouraud’s Dextrose Agar and counting colonies 48 h later. Mass reproduction was performed on grains of rice according to that described by Ángel-Sahagún et al.27
Field trial
Two experimental groups were formed with eight Holstein cows, each group was maintained in an area of 5 000 m2, separated from each other by 500 m. Both areas had ryegrass pasture (Lolium perenne), water tanks for drinking and natural shade provided by various native trees. The cows did not receive any treatment to control fly infestation during the year prior to this trial. The experiment began the first week of September, before the second fly population peak of the season according to information reported in the area.22,23 The first group received an application of the aqueous formulation of Ma134 fungi (1 x 108 conidia/ml concentration), water, Polyoxyethylene sorbitan monooleate (Tween 80®, Sigma-Aldrich Co., St. Louis, MO, USA) solution (0.01 %) and an agricultural adjuvant at 0.1 % (Inex-A, Cosmocel, Mexico). Five litres of the formulation were applied per animal by aspersion using a back sprayer with a cone-type nozzle at a pressure of approximately 40 lb/in2, on four occasions with seven-day intervals between treatments. The formulation was prepared 30 min prior to administration. The control group received a water solution with Tween 80 (0.01 %) plus an agricultural adjuvant at 0.1 %. Sprayings were between 7 and 8 pm to avoid sunlight. The number and interval of applications were similar to that used in a previous study with the stable fly.25 The Committee on Use and Care of Animals of the Instituto Tecnológico El Llano Aguascalientes approved this project, and adequate veterinary care was provided to all of the animals under study.
Estimation of H. irritans infestation
An Infestation index (average number of flies per animal) was estimated daily by the direct count of adult flies found resting or feeding on the animals; each cow was considered an experimental unit. This estimation consisted of photographing only one side of the body of each animal (including regions of the head, neck, back, sides and limbs). using a digital camera with a 5x optical zoom function. This activity was done daily between 8 and 9 am, always by the same person, who was not aware of the status of the cows. Counting the number of flies was performed by computer image analysis. The obtained number was multiplied by two to obtain an estimate of the total number of flies.20,28 Climatic factors were registered as reported by the El Llano weather station, the measurements included the average daily temperature (T °C), the average daily relative humidity (RH %) and the rainfall (Rf) during the study period.
Data Analysis
The information recorded in the groups under study (treated and control) was analysed by analysis of variance and Student’s t-test (p< 0.05), with the objective to demonstrate the differences between the groups in each study week using the corresponding week’s average index of infestation values. Then, Abbott’s formula was used to determine the efficacy of the formulation29 by identifying the percent reduction in the average infestation index for each evaluated week.20
Results and discussion
The population curve of the horn fly, H. irritans, in the groups under study is shown in Figure 1. On the second day post-treatment, the daily index infestation began to decrease compared to the control group, maintaining this trend until the end of the study. Infestation index at day 0 in the treated group was 530 and in the control group, 532. The aqueous formulation reduced the index infestation of 538, which was determined at the start of work (day 1), to 169 on day 28 post-treatment, representing 68.6 % efficiency.

Figure 1 Infestation index of horn flies, Haematobia irritans, on cows treated with the aqueous formulation of Ma134
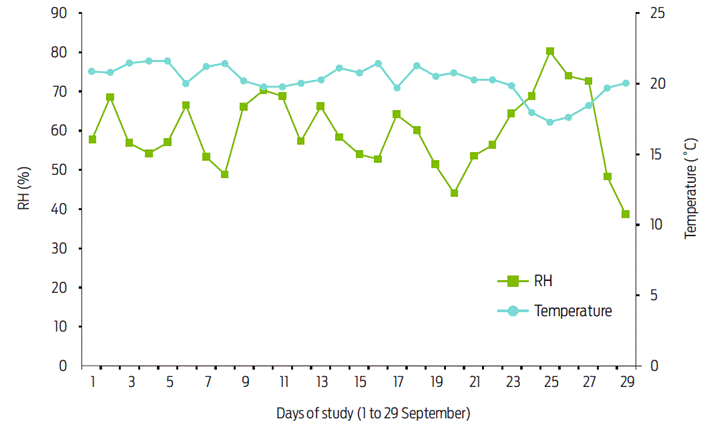
In Table 1, the average infestation index is shown in each of the study weeks. The infestation index was reduced from week to week in the treated group. In the control group, it was maintained between 530 and 569 in the first three weeks; however, in the fourth week, it was reduced to 479. The number of flies in the treated group was always less than in the control group. Statistical differences were detected each week between treated and control groups: first week (t 14= -3.14, p= 0.006), second week (t 14= -10-19, p= 7.3 x 10-8), third week (t 14= -25.88, p =3.17 x 10-13), and fourth week (t 14= -9.08, p= 3.04 x 10-7). The cattle remained healthy during the study period. Climatic data during the study period indicated that the average temperature was 20.2 °C with a range from 18 to 21.6 °C, while the RH was on average 59.7 % with a range from 38.4 to 80.3 %, and no rainfall was registered (Figure 2).
Table 1 Infestation index average and efficacy of the Ma134 aqueous formulation applied on Holstein cows naturally infested with H. irritans. Different subscripts (a,b) on the same row indicate significant differences (p< 0.05). Efficacy: reduction percentage of the average infestation index calculated with Abbott’s formula.

The results obtained in this study show that the formulation of the Ma134 strain was able to control horn fly infestation under field conditions. Compared to the control group, an infestation reduction of the fly in the treatment group was consistent during the study, and the weekly applications helped reinforce the pathogenic action of the fungus. Thus, it can be considered that the treatment had an additive effect. The aqueous formulation of Ma134 was added to an agricultural adjuvant to promote adhesion of conidia to the flies and animal hair and provide assistance in protecting the conidia from exposure to sunlight. In the literature, it is mentioned that conidia are sensitive to UV rays and to high environmental temperatures, and when applied to the body of animals conidia are sensitive to body temperature.30 The design of a formulation for topical application should consider at a minimum these factors; hence, the incorporation of an agricultural adjuvant or another compound with a heat/UV protecting ability can improve the performance of the conidia.21,27 However, Angel-Sahagún et al.27 did not find that environmental conditions had any effect on the performance of entomopathogenic fungi used in experimental grasslands artificially infested with larvae of Rhipicephalus microplus. The tolerance of entomopathogenic fungi to environmental conditions appears to be related to the site where the fungus was isolated,31 and it can be assumed that the Ma134 strain is adapted to a semiarid climate, the same as the study site, which probably contributed to its good performance in the study.
M. anisopliae has been the most studied entomopathogenic fungus because of its cosmopolitan and highly pathogenic nature. In addition to the fungus being specifically pathogenic for common insects affecting domesticated animals, such as ticks and flies, it is an organism that has minimum risk for plants, animals, humans and the environment.15,30,32 In this study, no adverse effects of the fungus were observed in the cattle. Other authors reported similar findings in cattle infested with ticks or stable flies and treated with M. anisopliae formulations under field conditions.25,33,34 The homogenous weather conditions in the study period and an adequate combination of humidity and temperature were ideal for the development and maintenance of the population curve of H. irritans. However, at the end of the study, the change in these environmental conditions, especially the low average daily temperature, favoured a decline in the infestation of flies. Cruz-Vázquez et al.22,23 describe similar population dynamics in the region, characterized by a close relationship with temperature and relative humidity. In these studies, it is mainly suggested that temperature induces a decline in population growth and in the late fall temperature can cause diapause.
Recently, in Tecoman, Colima, Mexico, a region with a tropical dry climate, the effect of several strains of M. anisopliae on steers with an experimental infestation of H. irritans was reported: mortality was observed from day 1 post-treatment (20 %), on day 4 it was 61 %, by day 8 it was 84 %, and from days 12 to 13, it was between 94 % and 100 %. However, the absence of reinfestation limited the possibilities of estimating the longevity of the effect of the fungus.20 In São Paulo state, Brazil, a region with a sub-tropical climate, a study was performed to evaluate the susceptibility of adult H. irritans flies to a water-based formulation of a strain of M. anisopliae under natural infestation conditions. For this purpose, the formulation had a concentration of 3 x 1010 conidia /ml and was applied to cattle every five days on four occasions. This study found that the level of infestation reduced in the treated group from the second spraying of the formulation, while in the untreated group infestation always increased. At the end of the study, the treated group had an average of 28.5 flies/head while the untreated group had 65.4 flies/head.21
In our study, an additive effect was also found. This phenomenon was observed both in cattle naturally infested with S. calcitrans25 and in cattle infested with R. microplus ticks under field conditions.34 The use of multiple applications of an entomopathogenic fungus provides better results.30,33 The results obtained in this study confirm the potential of M. anisopliae to infect and cause death in H. irritans under natural infestation conditions. Because the infestation by the horn fly in the region under study is seasonal, it would be advisable to start a control programme using this formulation when the first detection occurs to limit the fly population growth. Thereby, reducing its adverse effects on production and animal welfare, as well as the use of insecticides. This biological control is a safe and sustainable alternative that has minimum risk for vertebrates, humans and the environment.15,30 According to the literature, it is possible to use in combination with other biological control agents, such as parasitoids35 or insecticides.36 To our knowledge, there are no studies on the simultaneous use of different natural enemies or combinations with insecticides in the case of the horn fly. Nevertheless, to transfer this alternative of biological control to ranchers, more research is necessary including (1) a completion of more field trials with a greater number of animals in different conditions to further validate the biological control’s performance, and (2) an estimation of the cost-benefit ratio.
Conclusions
The results of this study demonstrated that the Ma134 formulation was 68 % effective in reducing infestation of the horn fly, H. irritans, on dairy cattle under natural infestation conditions. For this reason, the formulation can be considered a biological control for the integrated management of this insect. However, further investigation is needed to ensure its performance as a biological control.











 nueva página del texto (beta)
nueva página del texto (beta)