Introduction
Intensive pig farming entails a great danger regarding the presentation and prevalence of diseases, particularly respiratory ones. The swine influenza virus is one of many viral agents implicated in respiratory disorders in pigs.1 Consequently, the incidence of contagious bacterial disease increases, and both the disease itself and the associated costs of antibacterial drugs impose a burden on the profitability of production units and cause problems with vaccination protocols, i.e., a weaker immune response to vaccination.1,2 In America, infectious diseases generated by new influenza viruses are the main cause of pig morbidity in integrative production units.3 In similar scenarios, viral diseases are a threat in other parts of the world.1 It is known that vaccination may not protect against all the viruses circulating in a given area.1,3 In addition, current vaccines may not be effective in young pigs due to interference with antibodies received from the sow. Producers often need to vaccinate their animals after maternal antibodies decrease, that is, when they are 10-13 weeks old.4 A vaccine can completely protect against influenza if antibody titres against the challenge virus are sufficiently high, a relatively rare event.5 Apart from the vaccine strain, the adjuvants and antigenic dose may also play a crucial role in vaccine efficacy. Immune-modulating substances may help the animals achieve the high antibody titre required for full protection.1,3 Additionally, it has been postulated that drugs or products that can optimize immune responses may not only be beneficial to the health status of the farm but also improve production variables while maintaining a favourable cost:benefit ratio.6,7 In pigs, such a drug preparation should ideally be administered orally, be virtually innocuous to animals, and not pose a public health threat, i.e., drug residues in animal tissues should not be a concern.
Glycyrrhizic acid from liquorice is a potentially useful immune-stimulating active principle, particularly useful against influenza virus.8 It is already available in the human market, and it is commercialized as a nutraceutical preparation mixed with antioxidants, vitamins and oligoelements (Viusid®, from Catalysis, Spain; distributed by Dermaceutical México, SA de CV Mexico City). Glycyrrhizic acid has been shown to possess in vitro and in vivo antiviral activity that interferes with both DNA and RNA replication, thus interfering with the replication of a wide range of viruses, including herpes, influenza A and B, hepatitis B, coronavirus and SARS9-14 Glycyrrhizic acid and derivatives have been shown to be able to impede the release of virions from their capsids,14 apparently due to a dose-dependent inhibition of protein kinase P phosphorylation15,16 and perhaps due to its ability to decrease membrane fluidity.8 Likewise, glycyrrhizic acid interferes with arylamine N-acetyltransferase activity in bacteria, thus showing antibacterial effects against at least Streptococcus spp., Haemophilus spp., and Klebsiella spp.17-19 Supplementation of antioxidants, vitamins and oligoelements may also have positive effects when mixed as in the commercial preparation Viusid-Vet® powder (Dermaceutical SA de CV, Mexico City). However, these components may have less conspicuous effects than the ones already identified for glycyrrhizic acid, considering that modern diets for pigs contain the necessary vitamins and microelements required by this species. In broiler chickens, glycyrrhizic acid stimulates weight gain, and this effect is linked to immune-related effects.20 This was observed even in chickens affected by infectious bronchitis.21 Furthermore, glycyrrhizic acid augments the survival of shrimp affected with the viral disease known as white spot syndrome,22 and again, an immune-mediated mechanism is proposed. Hence, considering the above, the aim of this trial was to assess whether glycyrrhizic acid supplementation can stimulate the humoral immune response towards pig influenza, decrease viral shedding as assessed by PCR, and increase production variables in growing pigs.
Materials and methods
The experiment was conducted at the Centro de Enseñanza e Investigación en Producción Porcina (CEIEPP), a farm that belongs to the School of Veterinary Medicine at the Universidad Nacional Autónoma de México. The farm is a 200-sow full-cycle farm, located in the northern part of Mexico City in Jilotepec, State of México, 99°31´45” W 19°57´07” N, at 2 250 m above sea level. The weather is temperate and windy year-round, with a mean temperature of 18 °C and 608 mm of rainfall per year.
Outbreak of influenza and experimental animals
An influenza outbreak occurred approximately two weeks prior the commencement of this trial during the winter of 2013. The viral challenge was detected with qRT-PCR from nasal swabs, as described below. H1N1 virus isolation was carried out in 9- to 11-day-old SPF chicken embryos, thus confirming the cause of the respiratory signs observed in the pigs. Clinical signs included cough, nasal discharge, anorexia and fever (40 °C). The experimental groups were composed of pigs with respiratory clinical signs, and the chosen animals were not moved from the farm but only relocated to a different pigsty within the farm.
From unpublished previous experiences in other influenza outbreaks, sample size was calculated using G*Power.23 Two endpoints were chosen: the difference between the weight gains of the control and treated groups (values used: 0.554 and 0.570 for control and treated with ± 0.28 as SD) and the difference in proportion of control and treated pigs that tested positive for the H1N1 subtypes of porcine influenza (0.90 and 0.67 for control and treated). The sample size obtained from these parameters was 78; hence, the final number of pigs in this study was sufficient for statistical inference (80 piglets). The animals tested were male and female Duroc-Landrace cross piglets from a formerly influenza-negative farm. The males were neutered when they were three days old. When entering this trial, piglets were one day weaned and 22 days old, weighing approximately 7 kg (6.94 ± 0.4) kg. The litters were ordered according to mean body weight, and then systematic random sampling was used as a randomization tool. This implies that one of every three sick piglets was assigned to one group until it contained 40 individuals (20 males and 20 females). The same was repeated for the untreated control group.
Supplementation of glycyrrhizic acid
Glycyrrhizic acid was added to the feed at a rate of 0.024 kg of glycyrrhizic acid/ton of food, equivalent to 5.2 kg of the commercial preparation throughout this trial. The control group was fed as above, but with a glycyrrhizic acid-free commercial diet. The sows had been vaccinated against influenza virus, and all piglets were screened for influenza antibodies using haemagglutination inhibition (HI) tests on samples taken every two weeks from all animals, beginning on week two after the beginning of the trial and continuing until week fifteen.
Haemagglutination inhibition assay
A standard procedure from the World Organization for Animal Health (OIE)24 was used with the following modifications: standardization was performed using eight haemagglutinating units (HAU). The serum was heated to 56 °C to inactivate it, and then it was adsorbed with kaolin and chicken erythrocytes at 5 %. Serial twofold dilutions from 1:40 through 1:5120 were utilized. Titres were considered positive when they were greater than or equal to 1:80.24 Sampling of all animals was conducted on week two after the beginning of the trial and every fortnight until week fifteen.
Samples for detection of antibodies to influenza
A follow up-was established for porcine influenza virus (HI-IP), sampling all animals on week two after the beginning of the trial and on week fifteen. Additionally, antibody titration tests were performed using A/swine/New Jersey/11/76 (H1N1) (GenBank accession no. K00992) subtype A/swine/Minnesota/9088-2/98 (H3N2) (GenBank accession no. AF153234) as reference viruses. Pigs were considered positively exposed to influenza A virus if their HA-IP titres were at least 1:80 or 1.9 log. Animals with titres 1:40 or less were considered unexposed to HI-IP.
Samples for qRT-PCR
Additionally, quantitative qRT-PCR tests against the M gene were performed in all animals to assess viral shedding from nasal swab samples on weeks 1 to 8 after the beginning of the experiment. Viral counts are log-expressed, with values 2.0 or higher considered positive. A commercial real-time PCR was used (Find-IT Influenza, Cat. No. FI50 Bio-Genica, Montreal, Quebec, Canada). This kit can detect the M gene of the influenza virus using real-time One Step (TaqMan) PCR. The M gene confers species specificity. It has been reported that the M gene variants of human and avian viruses can be distinguished by several amino acid substitutions in both the Ml and M2 proteins; however, the M gene is more conserved than the H or N gene.
Productive parameters
All piglets and their feed were weighed every week. The food was weighed when offered, then the unconsumed feed was also weighed, and 10 % waste was assumed. Average daily gain (ADG) (g), weight gain (g), feed conversion rate (FCR) (kg) and conversion rates (kg:kg) were obtained only on week 18 due to technical reasons.
Statistical Analysis
To examine the immune and growth trajectories of each individual pig, generalized estimating equations (GEEs) were used to construct a statistical model. For immune results, the link function was eη, and the linear option was utilized to estimate weight (kg) parameters.25 Identification was used as a subject variable, and week as a repeated-measures between-subject variable. The GEE method requires that a correlation structure be established with the lowest possible mean squared error (MSE). The independent structure chosen had the lowest MSE (0.0450). The explanatory factors of gender and group (treated, control) were used to account for differences in growth between males and females due to the experimental treatments. Interactions between these variables were added to the model. On week 18, Student’s t-test was carried out between control and treated mean differences for daily feed consumption (kg), daily weight gain (kg) and conversion ratio (kg:kg). The statistical package IBM-SPSS 20® was used to test for statistical significance with α = 0.05 limit.
Formal scientific information on experimental treatments for an infectious outbreak of a disease are seldom published in veterinary medicine. The difficulty of preparing the trial in a timely manner, the potential costs involved and the statistical difficulties compared to experimentally induced infections explain this trend. Thus, various statistical models were counterbalanced for this trial, and it was concluded that to apply a suitable statistical treatment for this study, it should be regarded as a longitudinal one. Consequently, a GEE approach was adopted as the best model to analyse the data.25 GEE is an extension of the generalized linear model that takes into account the progress of each individual over time.25 The statistical significance of the model was assessed with the likelihood ratio test, which approximates a χ2 distribution with degrees of freedom (df) equal to the difference in the number of parameters between the null model and the model of interest.
Results and discussion
In the HI test used to detect antibodies against H1N1 tests on week two, the glycyrrhizic acid-treated group showed fewer positive responses compared to the control group (47.3 % vs 61.1 %). On week 15 for H3N2, the values were 15.8 % vs 22.2 % for the treated and control groups, respectively (Table 1). The cutoff levels for samples to be considered positive were HI titres of 1:80 or 1.9 log. On week 15 of the production cycle, the H1N1 and H3N2 blood sample tests showed a similar difference between the glycyrrhizic acid-treated group and the untreated control group (71.4 % vs 86.6 % for H1N1 and 0 vs 73.3 % for H3N2 in the treated and control groups, respectively).
Table 1 First and last results of the hemagglutination inhibition test using the H1N1 and H3N2 subtypes
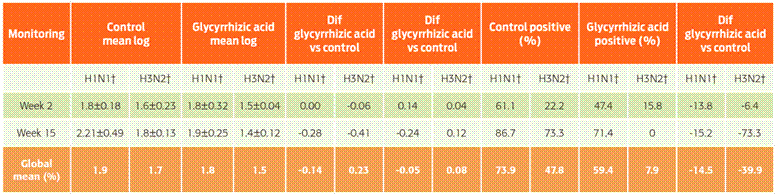
* Dif = difference given by H1N1 or H3N2 between control and glycyrrhizic acid groups for mean log titres and % positive samples. † Antibody titration tests were performed using A/swine/New Jersey/11/76 (H1N1) (GenBank accession no. K00992) subtype A/swine/Minnesota/9088-2/98 (H3N2) (GenBank accession no. AF153234), as reference viruses. *Titres that were 1:40 or less or a 1.9 log value were considered unexposed to HI-IP.
Figure 1 shows the results of the RT-PCR viral shedding test against the M gene, with an overall mean of 20.6 % positive for the treated group and 38.1 % positive for the untreated control group. This difference was statistically significant (χ2 1,7= 19.2; P = 0.0001). The glycyrrhizic acid group did not show viral excretion after seven weeks of treatment. Additionally, a lower positive rate in the glycyrrhizic acid group was observed after four weeks of treatment, considering the CT-value at 1.15.

(Likelihood χ2 1,7= 19.2 P = 0.0001).
Figure 1 Results of RT-PCR viral shedding test against the M gene.
Relevant data for the production variables assessed are summarized in Table 2 and Figure 2. The latter figure shows weekly marginal means ± DE for body weight in neutered male piglets and female piglets. Significant differences between groups were observed (χ2-likelihood test = 537.49, with df = 1; P = 0.0001) with the GEE for body weight. Additionally, we found a lack of statistically significant differences before week fifteen. However, on this week, the mean daily weight gain was higher in the treated group (1.038 ± 0.03 kg) compared to 0.637 ± 0.04 kg for the control group (T78= 34.27, P = 0.0001). This amounts to a 14 % higher daily gain, 14 % higher feed consumption and -0.4 % feed conversion rate for the glycyrrhizic acid-treated group compared to the control group. Additionally, the mean feed conversion rate for the experimental group was smaller than that of the control group (2.15 ± 0.61 and 3.97 ± 1.13, respectively) (T78= 5.7, P = 0.001), even though the experimental group had higher feed consumption values.
Table 2 Final values (week 18) obtained for weight gain, feed consumption, and conversion rates.
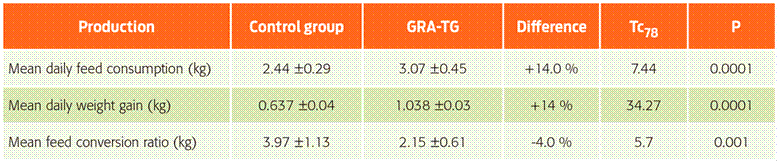
Tc78 = Student’s test (df = 78). † GRA-TG = glycyrrhizic acid-treated group
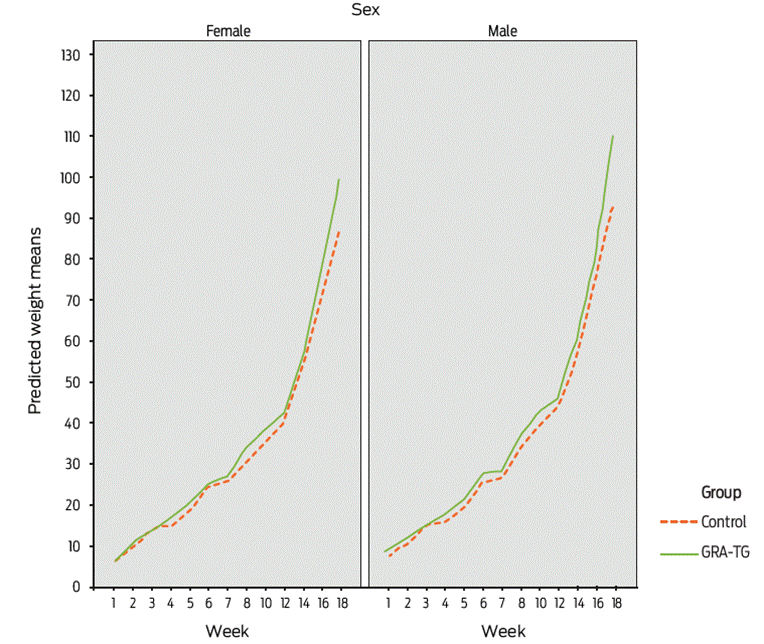
χ2 likelihood test1,11 = 537.4; P = 0.0001 in the GEE
Figure 2 Mean body weight weekly values obtained for glycyrrhizic acid-treated (GRA-TG) and control pigs, including female piglets and neutered male piglets.
The inclusion of various medicinal plants and their extracts and essential oils has been investigated for various purposes in pig farming, including inhibition of pathogens, modification of GI physiological status in healthy and diseased animals, increased activity of the immune system and growth promotion.6,7 Glycyrrhizic acid, a major triterpene glycoside isolated from Glycyrrhiza glabra L. (liquorice) and G. uralensis, is the leading natural glycoside and the chief sweet-tasting constituent of these herbs.26,27 Given the immunological findings obtained from the glycyrrhizic acid-treated pigs based on their H1N1 and H3N2 and considering that viral load for each virus type was not analysed in this study since only M gene was amplified, it can only be suggested that this preparation allows better resistance to the natural influenza viral challenge, compared to untreated pigs.
This trial was not designed to characterize the mode of action of glycyrrhizic acid as an antiviral agent. However, it is relevant to present some of its main effects. Utsunomiya et al.28 demonstrated that due to the ability of glycyrrhizic acid to induce interferon-γ, it exerted a strong protective activity on a model of lethal influenza infection in white mice inoculated with a high dose of the virus (ten times higher than the LD50). In influenza-infected human macrophages, application of glycyrrhizic acid resulted in a dramatic decrease of pro-inflammatory cytokine production.12 Glycyrrhizic acid has been shown to possess in vitro and in vivo antiviral activity interfering with both DNA and RNA replication.9-13 As in this trial, other authors have also proposed antiviral activity for glycyrrhizic acid.8,10-12,14 However, the precise manner in which this action is achieved is not known. The most recently proposed mechanism of action for this substance is its ability to decrease membrane fluidity, as this feature is necessary for the fusion of the viral envelope with the cell membrane in the course of the viral life-cycle.8 The results here obtained suggest that the constant administration of glycyrrhizic acid as Viusid-Vet® powder enhances the pig’s ability to reduce H1N1 and H3N2 viral load. However, on week two there were no differences in the immunological responses between treated and control groups, as assessed with HI. Statistically significant differences were only observed on week fifteen. This may be partly explained by the fact that the pig’s immune system is underdeveloped before postnatal day 56.29 Nevertheless, based on the fact that PCR revealed higher viral shedding in the untreated group, it can be proposed that the continuous administration of glycyrrhizic acid as in-feed supplementation with Viusid-Vet® powder allows a yet uncharacterized, immunological response that diminishes H1N1 and H3N2 viral shedding. A more detailed sampling is needed to define this response, as these results do not suffice to conclusively identify that glycyrrhizic acid induces a humoral immune response. Notwithstanding the above, glycyrrhizic acid, as presented in the commercial preparation in this test, improved production parameters in growing pigs, and again, it is tempting to link this effect to immune stimulation as poor weight gain is the main sign often observed after an outbreak of influenza.4,7,30 In any case, a direct economic benefit is expected with glycyrrhizic acid supplementation as Viusid-Vet® powder on farms affected with the influenza virus, particularly if the secondary expenses required to treat associated bacterial infections are taken into account.
The manner in which growth promotion is achieved is not clear. Glycyrrhizic acid has been recognized as a phytobiotic compound with antiviral and antibacterial properties,31 and these actions, together with the antioxidant effects of the Viusid-Vet® powder formulation, may partially explain the growth promotion observed. This effect has already been reported in chickens.20,21 Glycyrrhizic acid is known to stimulate gastrointestinal motility and enzyme secretion.32 Additionally, it has been shown to possess other pharmacological properties including anti-inflammatory, antiulcer, anti-allergic, immune-modulating and antiviral properties.33-36 These actions combined may explain in part the positive results obtained for the production variables assessed in this study, as the productive parameters on week 18 showed a 14 % higher daily gain in the glycyrrhizic acid-treated group, 14 % higher feed consumption, and -0.4 % feed conversion than the control group (Table 2). However, it is worth pointing out that the results obtained in this trial apply only to the commercial preparation utilized, i.e., Viusid-Vet® powder, as the effects of glycyrrhizic acid cannot be clearly separated from the combined antioxidant and metabolic actions of all the components of the commercial preparation (glucosamine, maltodextrin, arginine, glycine, ascorbic acid, pyridoxal, folic acid, calcium pantothenate, cyanocobalamin, malic acid, zinc sulfate, potassium sorbate and sodium benzoate). If only the production variables assessed in this trial are considered, the results can be viewed as encouraging for pig production, particularly because this active principle, as associated with the components in Viusid-Vet® powder, cannot be classified as a threat to human health and because the primary economic impact of this disease is related to retarded weight gain of pigs, resulting in an increase number of days needed to reach market weight.4
Swine influenza is a highly contagious viral infection that can be observed in farms as an epizooty or an enzooty.37 The commercial vaccines currently available do not protect against all influenza virus strains and emerging subtypes.38-40 In any case, vaccines provide a primary means to limit the clinical signs of influenza but may not be effective at blocking pathogen transmission and morbidity rates that usually approach 100 %. Mortality rates are generally low.29,37 Once swine influenza is established in a farm, it can be very difficult to completely eradicate it without complete depopulation. Thus, partial depopulation, segregation of early-weaned piglets, all-in all-out systems, and good hygiene practices are steps that can be implemented to control the incidence of the disease and minimize the economic impact on an affected farm. Given the results obtained in this trial, it appears that adding glycyrrhizic acid to the diet, as in Viusid-Vet® powder, can also be considered a logical step for some farms to address the aftermath of an influenza outbreak. A regular intake of 100 mg glycyrrhizic acid/day has been defined as the lowest-observed-adverse-effect level in humans.40 Using a safety factor of 10, a daily intake of 10 mg glycyrrhizic acid would represent a safe dose for most healthy human adults.41 In this trial, the end-cumulative dose of glycyrrhizic acid from Viusid-Vet® powder for each pig was approximately 10 mg/pig, with an approximate daily dose of 1 mg of glycyrrhizic acid/kg of body weight. Although this dose could be regarded as higher than the one recommended for humans, no side effects or toxicity of any form could be observed in the tested pigs. However, dose-response relationships and detailed animal toxicological studies for glycyrrhizic acid are warranted before this active principle can be considered for use on a commercial scale.
Conclusions
Feed supplementation with glycyrrhizic acid as found in Viusid-Vet® powder stimulates immune responses towards pig influenza, as measured by decreased viral shedding. Additionally, a positive response to viral protection, as assessed by HI titres, was observed at the end of the production cycle. Nonetheless, the results obtained in this trial are not conclusive evidence of immune modulation. Addition of this preparation to influenza-affected piglets improves their production parameters. Based on the tested immune response and considering the production variables assessed, economic benefits can be expected from glycyrrhizic acid supplementation as prepared in Viusid-Vet® powder. Further research is required to fully characterize cell immune responses to glycyrrhizic acid supplementation. Additionally, dose-response studies and toxicological analysis will be required to assess the full potential of this pharmaceutical preparation.











 nueva página del texto (beta)
nueva página del texto (beta)


