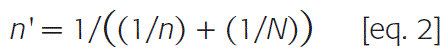Introduction
Poultry and swine bred in backyard production systems (BPSs) represent an important percentage of animal production activity, especially in developing countries. 1 Additionally, BPSs are recognized as a support economic activity, mainly in rural areas, stimulated by the increase in demand for organic and/or clean production systems.2 In general terms, BPSs can be characterized as possessing poor biosecurity conditions and low technological development for both the handling of animals and the optimal distribution of BPSs that improves animal husbandry.3 This situation generates a close contact between the backyard farmers and their families and the domestic animal species maintained at the location, such as poultry (hens, chickens, ducks and geese) and swine, pets, and wildlife. Thus, a high interspecies contact can be observed, increasing the risk of pathogen transmission between species.4-6 Evidence shows that this situation could also increase the pathogenicity and the host spectrum of pathogens, such as avian influenza virus and some serotypes of Salmonella spp.7-9
Characteristics of BPSs represent a permanent risk to the national health status. For example, transmission of pathogens between wild and domestic animals has been acknowledged to increase the risk of the spread and maintenance of avian influenza virus from migratory wild birds along their migration routes.10-13 Backyard poultry and swine are considered the main carriers of a number of strains or subtypes of priority zoonotic agents, such as avian influenza and Salmonella spp,11,14 becoming a zoonotic risk due to various factors including the direct contact between humans and sick animals and the risk of producing contaminated food (public health risk). Furthermore, a BPS may also undergo economic losses due to the high mortality of the affected species (e.g., highly pathogenic avian influenza).15 Despite its importance, there are few studies focused on this high-risk population stratum, and information about the sanitary statuses of BPSs in relation to the prevalence of infection with avian influenza, Salmonella spp. or any other zoonotic pathogen is scarce. The case of Salmonella spp. also presents a high level of underreporting due to the clinical signs and low severity associated with infections in both animals and humans.
Central Chile has the highest percentage of intensive productive poultry and swine establishments and number of animals. According to the last agricultural and forestry census in Chile, by 2007, there were more than 43.5 million poultry and 2.6 million pigs in the central zone, representing 83 % and 81 % of the total abundance of each species in the country, respectively.16 The number of BPS registered by the same date corresponded to 16,289 breeding birds and 2,282 raising pigs. Hence, with the lack of geolocation of these production systems, this study aims to prove the use of a spatial approach in the design and implementation of surveillance of zoonotic agents in cases where the location and distribution of the target population is unknown and to suggest a way to study these populations or those with similar characteristics.
Materials and methods
Target population
The present study was performed in three regions of central Chile: Valparaíso, Libertador General Bernardo O’Higgins (LGB O’Higgins), and Metropolitana de Santiago regions (Figure 1). According to the last Animal and Forestry census, in Chile, in the year 2007,16 16,289 BPSs breeding birds and 2,282 BPSs breeding pigs were reported in the study area (Table 1). The sample unit was defined as the BPSs that breed poultry and/or pigs that keep up to 100 birds or 50 pigs to survey for zoonotic agents.

Figure 1 A. Random sampling points by province assigned using ArcGIS® 10 and compatible zone detection by using free spatial tools. A. Study region with random sampling points. Study area and provinces: (1) Petorca; (2) Valparaiso, (3) Quillota; (4) San Felipe; (5) Los Andes; (6) San Antonio; (7) Melipilla; (8) Chacabuco; (9) Santiago; (10) Cordillera; (11) Talagante; (12) Maipo; (13) Cardenal Caro; (14) Cachapoal; (15) Colchagua. B. Random point (red pushpin) located in the Andes Mountains and 5 km searching area (yellow circle). C. Random point (red pushpin) and sampling candidate backyard farms (Yellow paddle) within less than 5 km.
Study design and sample size
A stratified and proportional random sampling was performed, based on the 15 provinces included in the study area. Sample size was calculated following equation 1 adjusted by equation 2, both extracted from Dohoo et al., 201017:
where
n |
= simple size; |
Zα |
= the value of Zα required for confidence = 1 - α, where α corresponds to the level of confidence; Zα is the percentile of a standard normal distribution (1- α/2); |
P |
= the expected prevalence of the pathogen (e.g., avian influenza, Salmonella spp.); |
q |
= (1 - p), and |
L |
= the precision of the estimation, also known as ‘allowable error’ or ‘margin of error’. |
where
n’ |
-= the adjusted simple size; |
n |
= the previous calculated simple size (eq. 1); |
N |
= the number of BPS in central Chile.16 Assuming a lack of knowledge about the prevalence of priority zoonotic agents present in BPSs located in central Chile, sample size was calculated based on a prevalence of 50 %, ensuring the maximum sample size possible when using a sample size approach for estimating a proportion,17 a confidence level of 95 %, and a precision of 5 %. BPSs raising pigs were used for the sample size calculation, supposing that they also breed poultry. Giving the final sample size of 329 BPSs distributed accordingly to each province, as detailed in Table 1. |
Random points allocation and validation
Random allocation of sampling points was performed using ArcGis 10 (ESRI 2011. ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute) according to the sample size established for each province (Figure 1). Sample points where checked for feasibility using Google Earth and Google Maps to guarantee the achievability of sampling at those points. Variables considered to identify a given point as a possible BPS were the presences of rural or semi-rural areas (small towns), nearby crops and houses or small towns. Points that did not fulfil these criteria were considered poorly positioned and were relocated. A radius of five kilometres was considered from the random sampling point, allowing for the possibility of sampling around points allocated on hills or in places with no livestock activity. Field validation was then performed to check for BPSs within the established radius.
Results and discussion
After the allocation of random sampling points for each province, the checking process showed that 251 (76 %) of the random points were well positioned in relation to the feasibility of finding a BPS, given the proximity to land with agricultural use, and 78 (24 %) sampling points were poorly positioned (Table 2). These poorly positioned points were allocated over lakes, within residential areas or within the Andes Mountains, and there was no chance of finding a BPS even within a radius of 5 kilometres. Details of the random sampling points and the distributions of well and poorly positioned points can be observed in Table 2. Poorly positioned points were re-allocated, removing the possibility of allocating points in the Andes mountains or those with the other conditions previously mentioned.
Table 2 Verification process for random sampling points and 5 kilometres criteria for each Province.
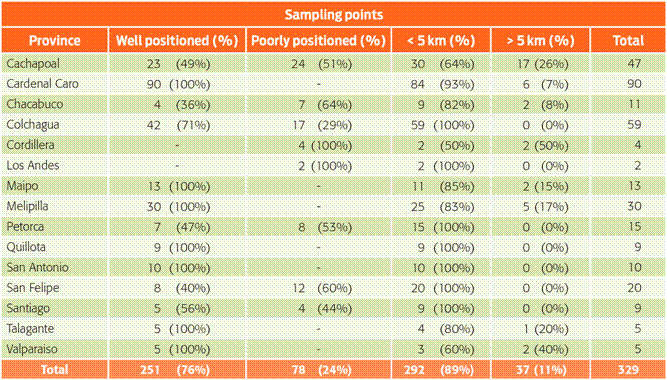
Provinces that presented poorly positioned points correspond mainly to those presenting a significant percentage of surface associated with the Andes mountains (Los Andes, Cordillera, Colchagua, Petorca, Cachapoal and San Felipe). In Chacabuco and Santiago, the number of poorly positioned points could be related to the use of land, which presents a trend to urban and agribusiness uses.18 Normally, land use consists of vegetable and fruit cultures, which increased the time spent finding a BPS to sample.19 This scenario should be quite different in the two other regions to be sampled, considering that the main activities in those regions are related to animal and agricultural farming activities.16
After adjusting the positions of the random points, it was observed that 89 % presented a BPS within a radius of 5 kilometres, and only for 11% was it hard to sample the 5 km radius. The most distant BPS was found approximately 13 kilometres away from the random point, a situation observed in the province of Talagante. Whereas, the closest candidate BPS was detected within less than 1 kilometre of the random point, also in the province of Talagante.
Given that there is a lack of knowledge about the specific location of each BPS that raises pigs or poultry or any other animals, there is a big loss of time resources and equivalent funds just on the detection of a feasible point.20,21 This increases the duration of the sampling activities and often results in requiring over an hour just to find one BPS feasible to be sampled, affecting the efficiency of field activities and the quality of the samples.21 Delays in field activities could affect the integrity of the sample, depending on the type of the sample and the agent involved.22,23 Previous work in our research group spent almost one day just to reach one allocated random point and then search for the closest BPS to sample (Di Pillo, F. data not published). According to the characteristics of these production systems, most birds or pigs are raised in partially enclosed systems, where animals are confined at night and released to an extensive rearing during the day, increasing the difficulty of sampling.24
The last agricultural and forestry census from 2007 in Chile underestimated the actual number of existing BPSs in the area, since it only registered those BPSs who perform tax payments associated with agricultural and livestock activities, leaving out of its records those who are without formal “agro” activities and maintain a low number of animals on their land. This scenario generates deficiencies in the design of surveillance programs, preventing an early warning of sanitary events occurring in this population stratum.25,26 Likewise, in the event of an emergency or a real-time outbreak, the time spent identifying and monitoring these populations becomes greater, affecting the health status and an early resolution of the event.20
Global tendency leads to the incorporation of spatial tools for the design and implementation of surveillance programmes and the storage of geo-coding data for animal health research.27 In addition, this may help to establish risk based surveillance programmes that will help to optimize time, human and budget resources for improving the quality of the programmes. The checking process becomes very important at the time of planning field activities, increasing the chance of visiting places with BPSs that are feasible to sample.
Conclusions
Normally, both surveillance and research activities have time and budget limitations, and thus, little information is known about BPS locations and dynamics, affecting the development of survey or surveillance programmes. The proposed approach would allow for an increase in the efficiency of sampling and field activities, decreasing the potential time spent and logistic resources used to locate a feasible sampling of BPSs compared to those needed for direct field inspection. More useful would be the generation of maps or census data with the specific locations of all BPSs in Chile.











 nueva página del texto (beta)
nueva página del texto (beta)