Introduction
Gastrointestinal parasites cause substantial health problems that undermine the production of grass-feeding ruminants. Parasites of the superfamily Trichostrongyloidea, particularly Haemonchus contortus and Teladorsagia circumcincta, impair productivity in sheep and goat-producing regions in tropical and temperate regions1,2. Control of these parasites has relied primarily on the intensive use of anthelmintic drugs such as benzimidazole drugs (BZ), primarily albendazole, oxfendazole, and fenbendazole; macrocyclic lactones such as ivermectin and imidazothiazole; and tetrahydropyrimidine derivatives such as levamisole and morantel-tartrate3. Nevertheless, with the continuous use of these anthelmintic drugs, anthelmintic resistance (AR) has developed in parasites4-10. In particular, the anthelmintic resistance AR of gastrointestinal nematodes (GIN) in sheep occurs globally and is described as a major threat to sheep production11,12. For example, gastrointestinal nematodes were resistant to benzimidazole on all farms assessed with a previous history of benzimidazole use in ithuanian sheep13, and in many flocks in India14. Moreover, AR reaches high levels in Asia, Latin America, Africa, Australia, and New Zealand, in addition to regions that rely on small ruminants as one of the primary sources of animal protein4-10. In Mexico, low resistance to benzimidazole was demonstrated in southeastern Mexico in 2003, but the authors warned that resistance could increase due to the continuous use of benzimidazole derivatives15. The warning was accurate, and AR in grazing ruminants is currently increasing in Mexico7,8,16. For the prevention and management of AR, important considerations are the frequency of the development of anthelmintic drug resistance and the extent at which it spreads17,18. In particular, for the benzimidazole derivatives used in sheep, the evaluation of their efficacy or the resistance to them is of paramount importance for the timely management of gastrointestinal nematodes14.
While studies on the mechanisms of AR indicate that the use of antiparasitic drugs that are in different chemical groups delays the selection of resistant nematodes1,19,20, Haemonchus spp. and Trichostrongylus spp. from sheep in Brazil have developed simultaneous resistance to albendazole, ivermectin, levamisole, moxidectin, and closantel21. Multiple-anthelmintic resistance has also been reported in major sheep producing countries, such as Australia and New Zealand, and in various South American regions1. Nevertheless, information is not available on the combined effects of benzimidazole drugs plus closantel to treat herds with resistant gastrointestinal nematodes. Therefore, in this study, we assessed the anthelmintic resistance of gastrointestinal nematodes in sheep treated with closantel (CLOS), albendazole (ABZ), fenbendazole (FBZ) and the combinations of CLOS + ABZ and CLOS + FBZ, in three different locations in Mexico.
Materials and methods
The study was conducted on sheep farms in three different states in Mexico during the rainy season. Farm (A) was in Texcoco, Estado de Mexico, which is a region classified as a temperate wet climate with an annual mean temperature of 15.2 °C. Farm (B) was in Hueytamalco, Puebla, which has a subtropical climate, no dry season and an annual mean temperature of 20.0 ºC. Farm (C) was in Tlaltizapán de Zapata, Morelos, which has a subtropical wet climate and an annual mean temperature of 23.5 ºC.
Animals and study design
The design of the study was a two-factor complete block experiment. The study adhered to the guidelines of the Institutional Committee for Use and Care of Experimental Animals of the Universidad Nacional Autónoma de México (UNAM). On the three sheep farms, namely, farms A (Mexico State), B (Puebla State) and C (Morelos State), more than 200 crossbred sheep were reared under a semi-extensive system in which the animals graze in paddocks for approximately 10 hours during the day and are penned for the night. These farms have a long history of GIN, and ivermectin and benzimidazole derivatives have been routinely administered for two consecutive years, which may have led to the development of anthelmintic resistance. Closantel was used regularly during the 1990s on the three farms, but the substitute triclabendazole replaced closantel 15 years ago for the prevention and treatment of fasciolosis. All animals were last treated for GIN three months before the study.
To confirm the natural infection of the animals, fecal samples were collected from a randomly selected sample of 240 sheep on each farm, seven days before the beginning of the study. Samples were examined by coproscopy using the fecal flotation technique to detect strongylid eggs22. Briefly, 3 to 5 g samples of feces were collected directly from the rectum and then were transported in an ice-cooled box and stored at 4 ºC until processed in the laboratory. The maximum interval between sampling and processing in the laboratory was 48 h. Fecal egg counts were performed using the McMaster technique, with a detection level of 20 eggs per gram of feces, EPG22-25. The GIN load from the McMaster technique determined the selection of grazing sheep, and only sheep infected with more than 200 EPG were included in the study23. On all farms, fecal samples were collected the day the anthelmintic treatments were administered, and on days 14 and 21 after treatment.
On the three farms, male and female sheep, approximately 3 to 6 months in age and weighing 13 to 28 kg with more than 200 EPG, were randomly assigned to one of five experimental groups (n = 28/group). One additional untreated control group was established on each farm (n = 28/group). Two lambs that were three-months of age and had not received a previous anthelmintic treatment were included in the study because these animals shed more than 200 EPG when they were screened before treatment.
Sheep in the untreated group received a placebo of 30 ml of water. In the experimental groups, single doses of anthelmintic drugs were orally administered as follows: fenbendazole at a dose of 10 mg/kg (FBZ); closantel at 5 mg/kg (CLOS); albendazole at 10 mg/kg (ABZ); closantel + fenbendazole at 5 and 10 mg/kg, respectively (CLOS + FBZ); and closantel + albendazole at 5 and 10 mg/kg, respectively (CLOS + ABZ). Active principles were kindly donated by PARFARM S. A, Mexico City. Suspension batches of albendazole, fenbendazole and closantel were prepared by slowly adding weighed amounts of polyvinylpyrrolidone K-30 to a mixture of polypropylene glycol and cold sterile water with continuous stirring until a homogeneous suspension was obtained. On all farms, the anthelmintic drugs were used within a month, originated from the same batches and were stored in a cool, dry and dark place. Before drug administration, all animals were weighed.
Larval culture
To determine the species composition and the most frequent gastrointestinal nematode helminthofauna in the sheep, a pooled fecal culture from each farm was prepared. Approximately 50 g of pooled feces from each group of sheep was mixed with sawdust, pieces of sponge and water. Cultures in plastic containers were covered with foil and maintained moist in a cabinet for 12 days at 27 ºC. Each culture was opened daily and mixed with a wooden spoon to increase oxygenation. When necessary, water was added. Third-stage larvae of GIN (L3) were recovered using the Baermann extraction technique for 12 hours22. The genus-level composition of the larval nematodes was determined by microscopic examination of the first 100 randomly selected L3 from the coproculture extractions, with the aid of taxonomic keys26.
Fecal egg count reduction test
For the fecal egg count reduction test (FECRT), the calculations to estimate the percentage mean reduction of egg counts with 95 % confidence intervals were performed in an Excel spreadsheet created by Angus Cameron (AusVet Animal Health Services for the University of Sydney). Calculations were based on the RESO© FECRT analysis program (Version 2.0 CSIRO, Animal Health Research Laboratory, PARKSVILLE, 3052. University of Sydney)27. According to the World Association for the Advancement of Veterinary Parasitology guidelines, resistance to an anthelmintic class occurs when the percentage reduction in EPG after treatment is less than 95 % and the lower limit of the 95 % confidence interval is less than 90 %. When one of these two criteria is met, AH resistance is suspected2,23,27,28. Recently, the inclusion of the upper 95 % confidence limit in the assessment of the anthelmintic resistance status was recommended because with this inclusion, the situations in which anthelmintic resistance is possible but not certain can be distinguished from those in which anthelmintic resistance is confirmed29.
Statistical analyses
The general percentage reduction in GIN eggs per gram of feces for the two time periods of 0-14 and 0-21 d was analyzed, and these reductions were used as endpoints. The design of the experiment was a two-factor complete block design; individuals were randomly assigned to groups (treatment i = 1, 2, 3, 4, 5, 6) on each farm, two sample times were examined (k = 14, 21), and the different farms were used as blocks (j = A, B, C). The endpoint general percentage reduction in GIN eggs per gram of feces data were normalized with arcsin transformation (√p). Endpoint data were analyzed with a univariate general linear model. Differences among means were determined with Bonferroni tests. For the general linear model, a threshold of P < 0.01 was used to reject the non-difference null hypothesis between factors or for the interaction. A threshold of P < 0.05 was used for Bonferroni tests. Marginal means, standard errors and 95 % CI were obtained with the least squares method. Analyses were performed with the IBM SPSS 20® statistical software package. Additionally, the most frequent genera of GIN that occurred pre-treatment are presented.
Results and discussion
Based on the morphometric identification of larvae, Haemonchus spp., Cooperia spp. and Teladorsagia spp. were the most abundant genera on all farms before the administration of the five treatments. However, Trichostrongylus, Oesophagostomum, Chabertia, Nematodirus and Strongyloides were also detected.
Before the experiment, anthelmintic resistance was 1831.25 ± 173.31 EPG (fenbendazole group); 1760.41 ± 56.71 EPG (closantel group); 1689.58 ± 142.56 EPG (albendazole group); 1410.41 ± 193.78 EPG (closantel + fenbendazole group); 1539.58 ± 198.85 EPG (closantel + albendazole group); and 1518.75 ± 163.45 EPG (control group). After experimental treatments, the control and treated groups were tested for resistance on days 14 and 21 (see Materials and Methods). Accordingly, susceptibility was confirmed for the closantel plus albendazole combination (CLOS + ABZ, Table 1) because the percentage GIN fecal egg output was reduced by 96.46 ± 3.04 % (day 14) and 96.88 ± 3.04 % (day 21). Resistance was demonstrated against fenbendazole (FBZ), closantel (CLOS), albendazole (ABZ) and the combination CLOS + FBZ treatments because the mean percentage EPG reduction was ≤ 95 %, and the lower confidence limit of ≤ 90 % on both sample days (14 and 21 days) was based on the FECRT and WAAVP guidelines23,28. On average, the fecal egg count in untreated sheep was 1850 EPG in Estado de Mexico, 1350 EPG in Puebla, and 1700 EPG in Morelos. Significant differences were observed for the group factor (F5,10 = 85.22; P = 0.0001) but not for the time period when samples were collected (F1,17 = 0.034; P = 0.86) or for the group-block interaction (F10,17 = 2.27; P = 0.066).
Table 1: Marginal means and 95 % CIs of the percent reduction of eggs per gram at 14 and 21 days after treatment of sheep (n = 28 per flock) with fenbendazole (FBZ), closantel (CLOS), albendazole (ABZ), closantel + fenbendazole (CLOS + FBZ) and closantel + albendazole (CLOS + ABZ) on three different farms (A, B, C).
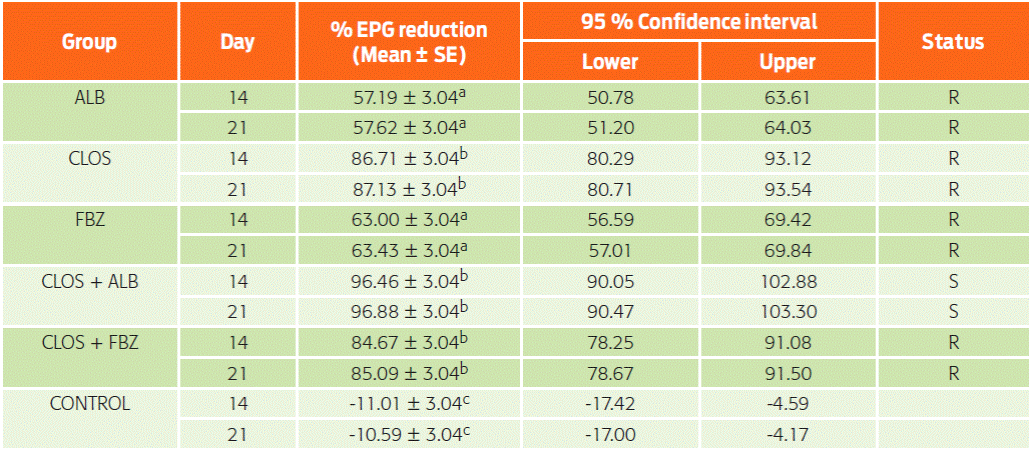
% EPG reduction = percentage reduction in eggs per gram CI = confidence interval R = resistant S = susceptible Superscript letters indicate Bonferroni differences (Bonferroni P < 0.05) among treatments (F5,10 = 85.22; P = 0.0001). Differences were not significant in the comparisons of day 14 and 21 (F1,5 = 0.034; P = 0.86).
Although no differences were found between days 14 and 21, the WAAVP guidelines indicate that samples must be collected 8-10 or 14 days after treatment. The general marginal mean and standard error with a 95 % CI on day 14 for the percent GIN reduction of EPG for each group are presented in Table 1. Figure 1 shows the arithmetic means ± SD of these data. The advantage of the fecal egg count reduction test (FECRT) is that it can be used for all anthelmintic classes; thus, the FECRT was appropriate for the categorization of resistance in this study. Moreover, according to recent studies, corrections in formulas and models can ensure categorization of resistance in small flocks or herds when the parasite population is highly aggregated or when animals excrete low counts of eggs24,30-33. Based on the FECRT following WAAVP guidelines23, GIN were susceptible to the combination closantel plus albendazole. This result was expected because a mixture of two or more actives with different pharmacological properties but a similar spectrum can increase anthelmintic efficacy or delay the development of resistance34,35. This beneficial effect is particularly noted following oral administration of combinations of different anthelmintic classes36,37. Fitness costs are associated with resistant genotypes, and nematodes that carry multiple sets of resistance genes are less fit than those that are resistant to only one anthelmintic class38. Thus, the evidence continues to increase that the best approach for the efficient control of GIN with current compounds and to even slow the development of resistance is to formulate them as combinations because the survival of resistant genotypes is minimized39,40. Nevertheless, these results must be carefully considered; although control is provided by using anthelmintics containing multiple actives, parasite control also increases when high levels of refugia are maintained35.
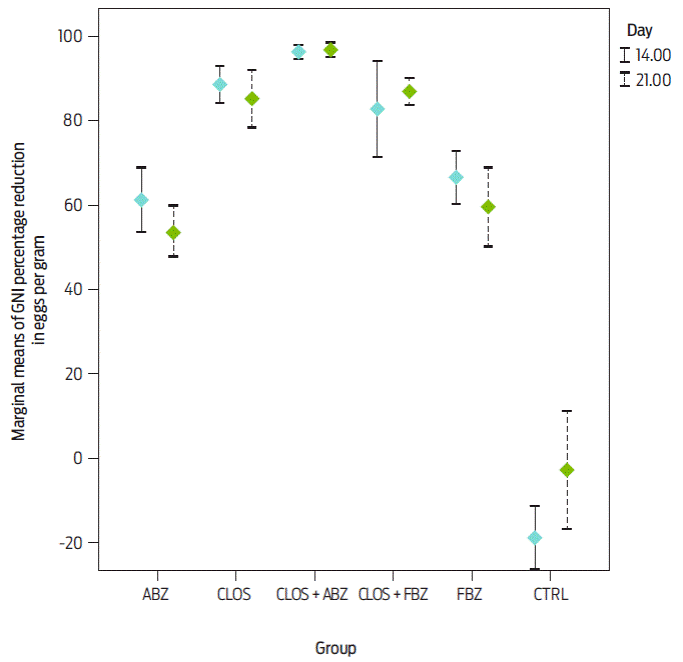
Figure 1: Marginal means ± SD of the percentage reduction in egg count on days 14 and 21 after treatment of sheep on three farms (n = 28 per flock) with fenbendazole (FBZ), closantel (CLOS), albendazole (ABZ), closantel + fenbendazole (CLOS + FBZ) and closantel + albendazole (CLOS + ABZ).
Two questions remain to be answered: (1) What is the mechanism of action to explain the susceptibility of gastrointestinal nematodes to the combination of closantel plus albendazole? (2) Why are GIN resistant to the treatment with closantel plus fenbendazole? To explain the efficacy of closantel plus albendazole, ATP synthesis may be strongly reduced because of degenerative changes in the endoplasmic reticulum induced by albendazole41 and the suppression of succinate dehydrogenase and fumarate reductase activities by closantel42. However, this proposed mechanism requires further investigation. Additionally, compared with almost no uptake of fenbendazole43, the higher bioavailability of albendazole provides a plausible explanation for the higher efficacy of albendazole. The bioavailability of albendazole is higher than most benzimidazole derivatives, and the parent compound and the active metabolites undergo biliary excretion and accumulate in the gastrointestinal tube44. However, further studies are required to explain the differences in the efficacy of these compounds.
In geographical areas in which intensive sheep production requires the continuous use of antiparasitics, the patterns of anthelmintic resistance observed in this study are commonly reported3,10,7,8,15,45. However, studies investigating such patterns of resistance are rare in Mexico. Thus, to decrease the selection pressure that leads to the development of anthelmintic resistance in ruminants, approaches to nematode control in sheep flocks must be based on epidemiological studies, fecal examinations, nutrition strategies and targeted, selective treatments, as suggested previously7,45,46.
Conclusions
In sheep naturally infected with gastrointestinal nematodes, anthelmintic resistance was determined after the administration of either single drugs that have different mechanisms of action or a combination of these drugs; namely, albendazole, fenbendazole and closantel. Based on this study, the combination of closantel plus albendazole can be effective for the control of infections by resistant gastrointestinal nematodes.











 nova página do texto(beta)
nova página do texto(beta)



