Introduction
Mammary cancer is the most common malignant neoplasm in intact dogs. Canine mammary tumors (CMTs) can vary in size from a few millimeters to several centimeters; they can exist in multiples (at least in 50% of the cases) and usually involve the caudal glands (Wey et al., 2000). Histologically, CMTs are classified as malignant epithelial neoplasms (carcinomas), special types of carcinomas, malignant mesenchymal neoplasms (sarcomas), carcinosarcomas and benign neoplasms (Goldschmidt et al., 2011).
Some risk factors associated with mammary gland tumors in dogs include gender and breed; it is well known that females are predisposed to the disease due to the tropism of natural estrogens for the mammary gland (Yu et al., 2002). Epidemiological studies often report a higher susceptibility in small-size breeds, although the number of reports in large-size breeds is increasing. The most frequently affected breeds include Poodle, Maltese, Chihuahua, Beagle, Yorkshire Terrier, Bichon Frisé, Cocker Spaniel, Springer Spaniel, Irish Setter and German Shepherd, among others (Bronden et al., 2010). In a retrospective study of biopsies of lesions in the mammary gland of female dogs, Salas et al. (2015) observed the epidemiological characteristics of canine mammary tumors and concluded the following: (1) mammary tumors in intact dogs had a high incidence (16.8%); (2) benign and malignant tumors had similar frequencies (47.7% vs. 47.5%, respectively); (3) epithelial tumors were the most common; (4) the most commonly affected dogs were old adult females (9 to 12 years old), purebreds (Poodle and Cocker Spaniel) and small-size dogs; and (5) there was an increase in malignant tumors in the last four years of the study. Therefore, mammary tumors in dogs are an important animal health problem that needs to be solved by improving veterinary oncology services in Mexico.
The increasing number of neoplastic conditions found in veterinary medicine, including mammary neoplasms, justifies further research efforts in the field of veterinary oncology. In this regard, it is necessary to develop studies that correlate histological features with a clinical outcome and to establish the most significant risk factors associated with the development and clinical behavior of canine mammary tumors. This development would provide valuable information that could be used to improve the diagnosis, prognosis and treatment of CMT patients. In addition, this information could be compared to data from other countries. It is also important to identify molecular markers that may help to achieve more accurate diagnoses, provide prognostic information, and offer better treatment options for canine patients with mammary cancer. In turn, because dogs with spontaneous mammary tumors have proven to be an animal model par excellence (Gama et al., 2008; Cassali et al., 2006), defining the most significant clinicopathological features of CMTs may offer valuable information for the study of breast cancer in women. We hypothesized that there is an association among histological features, risk factors and survival in female dogs with mammary tumors.
Material and methods
Eighty patients with mammary tumors undergoing lumpectomy or mastectomy at the Teaching Animal Hospital in FMVZ-UNAM from February 2012 to October 2013 were included in this study with the consent of their owners. Sampling was performed by convenience, as the typical cases were selected (Casal et al., 2003).
Because most patients had more than one tumoral mass, a total of 178 different mammary tumors were analyzed. To calculate the relative risk (RR), 80 patients over 4 years of age were randomly selected from hospital files, and these patients did not have a medical appointment not related to a mammary neoplasia during the same period of time were added to this study. Information on age, breed, body condition score and gynecological data (OVH and parity) was obtained from these patients.
The number and size (largest dimension) of tumors was obtained from each patient. Tissue specimens were fixed for 48 hours in 10% buffered formalin; they were trimmed, processed, embedded in paraffin, cut at 5 mm and stained with hematoxylin and eosin (H&E) (Thomas, 1995). Histological sections were reviewed and classified according to the grading of canine mammary tumors proposed by Goldsmith et al. (2011), which includes histogenesis, differentiation and anaplasia, and evidence of local invasion (stromal and vascular). Histological classification was established by the consensus of three experts. Risk factors included age, breed, body condition (based on a scale of 1 to 5, where: 1 = emaciated, 2 = underweight, 3 = ideal, 4 = overweight, and 5 = obese) and gynecological data.
To determine the biological behavior of the tumors, these 80 patients were followed up with regard to their general health status, evidence of recurrence or metastasis, and survival every 6 months during a period of 18 months after the initial surgery (patients were excluded if they died from suggestive causes of mammary disease, based on evidence of metastasis from clinical data. Some of the follow-up patients had X-ray analysis, others had X-rays and ultrasounds, and other patients were only examined physically.
Statistical analyses were performed using IBM SPSS Statistics 22 (IBM Corporation, 1994- 2016, USA) and consisted of a series of univariate analyses and frequencies of the different variables, bivariate analysis with the Chi Squared test (χ2), relative risk and Kaplan Meier survival analysis. A P < 0.05 value was considered statistically significant. A multiple correspondence analysis (MCA) (Costa et al., 2013) was performed between the biological behavior of the tumor and the dogs’ breed (both purebreds and mixed-breeds were included). The graphs were built using the Prism 6.0 package (GraphPad Software, Inc., USA)
Results
Number and size of tumors
From the 80 dogs studied, 55 (68.7%) had multiple mammary masses in different mammary glands, whereas only 25 of them showed solitary tumors (31.3%). In total, 178 different tumors were analyzed. In most cases, the dogs had between 2 (59/178; 33.1%) to 3 tumoral masses (46/178; 25.8%), and a few patients showed 11 (5/178; 2.8%) to 18 (4/178; 2.2%) masses. Of these 178 tumors, 104 (58.4%) were benign tumors, and 74 were malignant (41.6%). In addition, it was observed that single malignant tumors (15/74; 20.3%) were more common than single benign tumors (10/104, 9.6%) (χ2 P < 0.04).
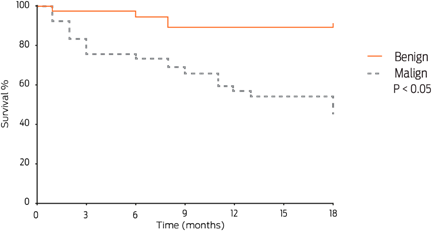
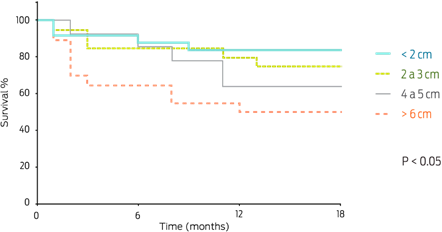
The sizes of the major tumoral masses varied; the most frequent size was < 3 cm (104/178; 58.4%), which was followed by tumors > 6 cm (43/178; 24.2%) and tumors between 4 to 5 cm (31/178; 17.4%). The biological behavior and size of the tumoral masses correlated, showing a highly significant difference (χ2 P < 0.05), with a trend for benign neoplasia < 3 cm and for malignant neoplasia > 6 cm (Figure 1).
CMT morphological classification. The histological analysis of the mammary tumors per dog showed a higher frequency of benign than malignant tumors; however, most of the dogs had multiple tumors, some of which were benign and some were malignant. We noted that more patients had at least one malignant mammary tumor (48/80; 60%), whereas those with only benign tumors were fewer (32/80; 40%).
Figure 2 shows the frequency of the different histological tumor types that were found. The most frequent benign mammary tumor was the complex adenoma (CA) (42/104, 40.4%), which was followed by benign mixed tumors (BMT) (30/104, 28.8%). On the malignant side, the most frequent tumors were simple carcinomas (SC) (18/74, 24.3%), complex carcinomas (CC) (11/74, 14.9%) and mixed-type carcinomas (MTC) (9/74, 12.6%). Moreover, the analysis of the CMT frequency based on the tissue of origin produced a similar occurrence of mixed and epithelial tumors. According to the biological behavior of the CMT, the mixed-type neoplasm was the most frequent (67%) of benign tumors; malignant tumors tended to be mainly epithelial (51%), showing a highly significant difference between groups (χ2 P < 0.05).
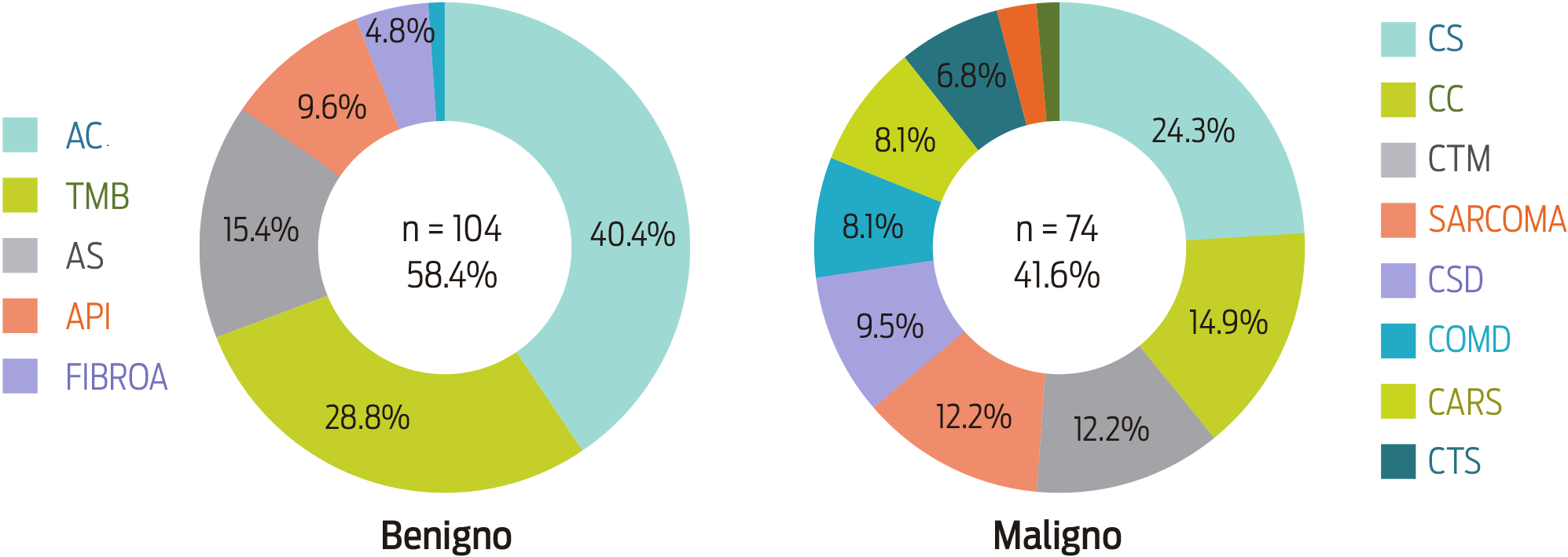
Figure 2: Frequency of canine mammary tumors according to their morphological classification. CA: complex adenoma; BMT: benign mixed tumors; SA: simple adenoma; IPA: intraductal papillary adenoma; FIBROA: fibroadenoma; SC: simple carcinoma; CC: complex carcinoma; MTC: mixed type carcinomas; SARCOMA: sarcoma; SDC: solid carcinoma; COMD: comedocarcinoma; CARS: carcinosarcoma; STC: special type carcinomas.
Risk factors associated with CMT
Age: a wide range of ages was observed in patients with CMT (3-16 years); the highest frequencies were for 8 (20%), 12 (16.3%), 11 (13.7%), and 9 years old (10%). In general, the most affected patients were dogs > 7 years old (67.5%), and they showed both benign (50%) and malignant CMTs (50%). In dogs > 13 years old, the ratio aligned towards malignant CMTs (92.3%), with a moderate risk of developing CMT and a higher risk that these tumors were malignant (χ2 P < 0.05) (Table 1).
Table 1: Relative risk (RR) of mammary canine tumors according to age, size, breed and parity.
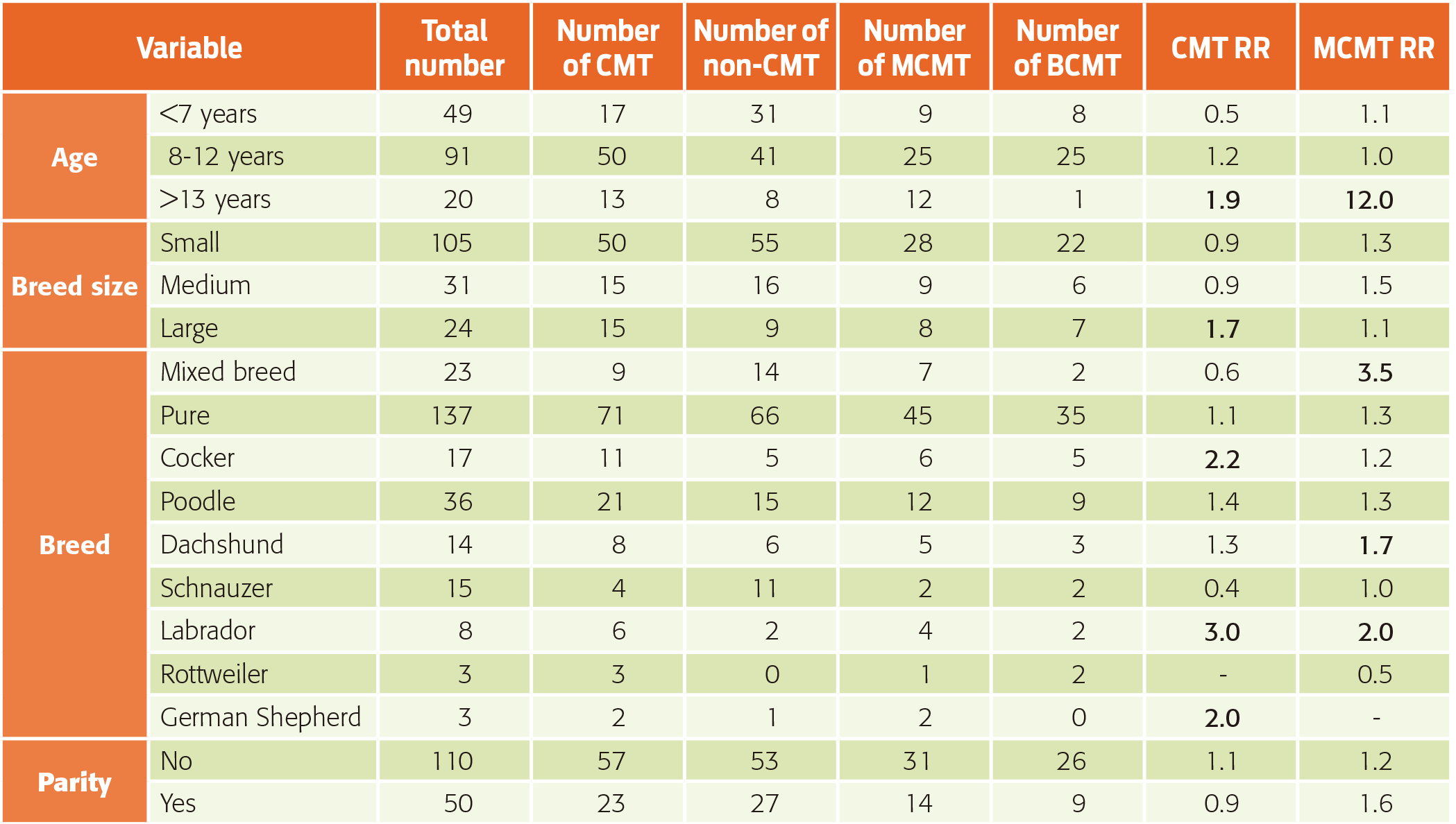
CMT: canine mammary tumor; MCMT: malignant canine mammary tumor; BCMT: benign canine mammary tumor.
Breed: As Table 1 shows, a wide variety of breeds affected with CMT was observed. Although purebred dogs had a higher frequency than mixed breeds, a significant relative risk predisposing them to the disease was not evident. However, mixed-breed dogs showed a higher risk of malignant tumors. In relation to the size of purebred dogs, the most affected were the small- and medium- size dogs, which did not show a risk or it was a non-effective risk disease. Among purebreds, the Poodle was one of the most frequently affected breeds, although its risk of disease was relatively low; the Cocker Spaniel had a moderate risk; the Dachshund showed a moderate risk for malignant tumors, although it had a lower CMT risk; the Labrador Retriever had the least frequency of occurrence, but it showed a higher risk of mammary tumors and a moderate risk of malignant mammary tumors; the Rottweiler and the German Shepherd showed a moderate risk of developing CMT (Table 1).
Gynecological data
Almost none of the patients were neutered within the first year and a half (78/80, 97.5%), and most of them were ovary-hysterectomized at the time of mastectomy. It was also observed that most dogs with mammary tumors were nulliparous; however, this condition had a negligible risk for CMT (Table 1).
Body condition score
At the time of surgery, 53.8% (43/80) of the dogs studied showed a body condition score (BCS) of 3; 28.8% (23/80) had a BCS of 4; 10% (8/80) had a BCS of 5; and 7.5% (6/80) had a BCS of 2. None of the dogs had a BCS of 1. By relating the body condition of patients to the biological behavior of mammary neoplasms, 51.2% (22/43) and 48.8% (21/43) of the dogs with a BCS of 3 had benign and malignant tumors, respectively, whereas 73.9% (17/23) of the dogs with a BCS of 4 tended to have malignant tumors, although the difference was not statistically significant (χ2 P > 0.05).
Recurrence and survival
Eighteen months after surgery, 15/80 (18.8%) patients had new tumoral masses. Of these patients, 7/15 (46.7%) had benign tumors, and 8/15 (53.3%) had at least one malignant tumor (P > 0.05). The recurrence of benign and malignant tumors was proportionally similar. Twenty-four out of 48 patients (50%) with at least one malignant mammary tumor, died; vascular infiltration was observed in 18/24 (75%) of these patients, and 14/18 (77.8%) died within the study period (P < 0.05).
Patients with malignant mammary tumors had a significantly shorter survival time than those with benign tumors (Figure 3). Of the patients with malignant mammary tumors, 19/74 (25.7%) died during the post-surgical follow-up; of these, 89.5% (17/19) exhibited an early death or died within a year. On the other hand, patients with tumors < 2 cm had higher survival proportions than those with tumors > 6 cm. Tumor size could thus be considered an important prognostic factor (P = 0.009) (Figure 4), as the following data shows: patients with CARS, COMD, and CSD tumors had a survival time of less than 6 months (2, 5.5, and 6 months of median survival, respectively), whereas patients with SC, CC, and MTC tumors had a median survival time of 11 months (Figure 5).
Discussion
Similar to our findings, other researchers observed that tumor size is an important prognostic factor: small mammary masses tend to be benign, and larger tumors tend to be malignant. In our study, patients with tumors < 2 cm showed a higher survival period than those with tumors > 6 cm. Ferreirai et al. (2009) found that 80% of benign tumors were < 5 cm, whereas 87.1% of malignant tumors were > 5 cm, which emphasizes the importance of evaluating tumor size; this variable is inexpensive and simple to assess routinely. Similarly, Ressel et al. (2013) found that mammary carcinomas showed a larger diameter (8 cm) than adenomas (3 cm); a lower survival time was found in dogs with tumors > 5 cm.
Oliveira et al. (2003) reported that mammary tumors represent approximately 50% of neoplastic diseases in female dogs, and 41 to 53% of these are malignant (Fonseca and Daleck, 2000). In our study, 58.4% were benign tumors, and 41.6% were malignant tumors, which is similar to the observations of Salas et al. (2015), who performed an 11-year retrospective study (2002-2012) in Mexico City. In this study, 47.8% of the total of mammary tumors were benign, 47.5% were malignant and 4.7% were non-neoplastic mammary lesions (hyperplasia, dysplasia or mastitis).
Regarding histological variants, similar to this study, other researchers found that the most common benign variants include mixed tumors and complex adenomas. The most common malignant variants were carcinomas that originated with a previous benign tumor, simple carcinoma and complex carcinoma (Im et al., 2014). In addition, Ressel et al. (2013) observed that complex adenomas were among the most common benign lesions, and simple carcinoma was the most common malignant variant.
Regarding the risk factors associated with mammary neoplasia, our results showed similarities with other epidemiological studies that had reported a wide age range in CMT patients. In our study, the range was from 3 to 15 years, with an average age of 8 to 12 years. Similarly, Kuldip et al. (2012) reported an age range from 2 to 16 years for mammary tumor development in dogs, with an average age of 9 years. The higher incidence was in dogs between 10 to 12 years old (31.37%). In addition, Zatloukal et al. (2005) found that dogs over 13 years of age showed the highest risk for the development of malignant tumors.
The breed predisposition reported for CMT is very diverse. In this study, the most commonly affected animals pertained to a small-size breed; however, the occurrence of mammary tumors has recently been increasing in large-breed dogs according to some authors, probably as a result of breed popularity in different geographic areas (Bronden et al., 2010). Rivera et al. (2009) reported that in Sweden, English Springer Spaniels showed a 36% predisposition for mammary cancer. Egenvall et al. (2005) reported a high incidence for mammary tumor development in Spaniels, Dobermans, German Shepherds and Boxers (Egenvall et al., 2005).
It is well known that dog breed popularity varies according to each country, which may influence breed predisposition analyses (Salas et al., 2015); however, we identified a moderate relative CMT risk in Cocker Spaniels. In addition, Dachshunds and mixed-breed dogs showed an increased risk of developing malignant mammary tumors. These breeds were also identified to be predisposed for CMT in a retrospective study performed in Mexico City using data from canine patients from the last decade (Salas et al., 2015). In a retrospective study conducted in the Czech Republic from 1977 to 2001, Zatloukal et al. (2005) reported that Poodles, Schnauzers, Cocker Spaniels, and Dachshunds were predisposed to developing mammary neoplasia. On the other hand, these authors did not find any CMT risk in large-breed dogs. This finding was contrary to our study in which we found a risk of disease even though those breeds are less popular. Furthermore, small-size breeds, particularly Poodles, did not show a significant risk despite being the most frequently affected breed. This demonstrates that a high CMT frequency in a popular breed does not necessarily indicate high breed susceptibility.
Several authors have reported an association between obesity, particularly in early life stages, and the development of mammary cancer in bitches (Sonnenschein et al., 1991; Perez et al., 1998). We found that most fat dogs had malignant neoplasms; however, there was not a significant difference in cancer development between dogs with a healthy body condition and fat animals.
It appears that exposure to endogenous ovarian hormones early in life is the most important cause of mammary tumor development in dogs, and the protective benefit from ovarian hormonal ablation seems to be dose-dependent. This means that the protective effect of ovariohisterectomy is strongest in patients with no exposure to minimal hormonal exposure and diminishes quickly after the first few years of life. Estrogen can be activated by epoxidation, thus gaining the ability to damage DNA and initiate mammary carcinogenesis (Yu, 2002). None of the patients analyzed in our study were neutered before their first estrous, which allowed their mammary tissue to be under a significant hormonal influence.
In this study, most CMT patients were nulliparous; however, contrary to the belief that has been held in veterinary medicine and has been tested in women, this variable was not a predisposing risk factor for the disease, which overthrows the myth that dogs must gestate to decrease the incidence of mammary tumor. It is well known that women who give birth and breastfeed for 3 months or more have a reduced risk of developing breast cancer; therefore, breastfeeding and term pregnancy have a protective effect against breast cancer (Rojas, 2008).
In this study, we observed that vascular invasion and the presence of neoplastic emboli in the lumen of lymphatic and blood vessels were poor prognostic factors. Chang et al. (1995) found a correlation between the presence of neoplastic emboli and a survival time between 18 to 24 months in dogs with CMT. Furthermore, Diessler et al. (2009) observed that vascular invasion can be used as an important CMT prognostic value.
The histological type is an important independent prognostic factor for CMT. Benjamin et al. (1999) reported fatal outcomes in Beagles associated with ductular carcinomas (62.5%) and adenocarcinomas of other origins (27.4%; solid, tubular, or papillary carcinomas; simple or complex). They also evaluated that in complex carcinomas, the rate of recurrence was 3.8%, and the rate of metastasis was 43.4%; for simple carcinomas, the numbers were 4% and 52%, respectively; and for ductular carcinomas, the numbers were 4.3% and 59.6%, respectively. The rate of metastasis for carcinosarcomas was 100%. In our study, the worst prognosis was observed in solid carcinomas, comedocarcinomas, and carcinosarcomas in decreasing survival time order. On the other hand, the least aggressive types were simple carcinomas, mixed carcinomas, and complex carcinomas; the latter histologic type showed the longest survival time. This observation coincides with a previous report that showed that complex carcinomas exhibited a low rate of local invasion and lymphatic permeation (Misdorp and Hart, 1976).
Conclusions
In the present study, the majority of patients had multiple mammary masses, and most tumors (58.4%) were benign. Most patients with multiple neoplastic masses had at least one malignant tumor. Canine mammary tumors often affected dogs older than 8 years of age, and geriatric dogs tended to present malignant tumors. Commonly affected breeds included Poodles, Cocker Spaniels and Dachshunds, but only Cocker Spaniels presented a significant risk for mammary tumor development, which demonstrates that the most commonly affected breeds are not necessarily the most susceptible. In this study, the increased risk of disease in nulliparous dogs was not corroborated. Mortality was the highest and the survival periods were the shortest in patients bearing malignant tumors larger than 6 cm. The shortest survival periods were also noted in animals with solid carcinomas, comedocarcinomas, and carcinosarcomas, in decreasing order.











 texto en
texto en