Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Veterinaria México OA
On-line version ISSN 2448-6760
Veterinaria México OA vol.2 n.1 Ciudad de México Jan./Mar. 2015
Research note
In vitro predatory activity of Lasioseius penicilliger (Arachnida: Mesostigmata) against three nematode species: Teladorsagia circumcincta, Meloidogyne sp. and Caenorhabditis elegans
Noemí García Ortizª 0000-0002-6935-0709, Liliana Aguilar Marcelinob* 0000-0002-8944-5430, Pedro Mendoza de Givesb 0000-0001-9595-3573, María Eugenia López Arellanob 0000-0002-8688-4411, Carlos Ramón Bautista Garfiasb 0000-0002-3359-8809, Roberto González Garduñoc 0000-0003-0333-7787
a Universidad Autónoma del Estado de Morelos, Facultad de Ciencias Agropecuarias, Av. Universidad 1001, Col. Chamilpa, 62209, Cuernavaca, Morelos.
b Centro Nacional de Investigación Disciplinaria-Parasitología Veterinaria, INIFAP Instituto Nacional de Investigaciones Agrícolas, Forestales y Pecuarias, Carretera Federal Cuernavaca-Cuautla No. 8534, Col. Progreso, 62550, Jiutepec, Morelos. * Corresponding author: Liliana Aguilar Marcelino. Tel: + 52-77-7319-2860
c Unidad Regional Universitaria Sursureste, Universidad Autónoma Chapingo, Km 7.5 Carr. Teapa-Vicente Guerrero 86800, Teapa, Tabasco.
Received: 2014-06-06.
Accepted: 2015-02-19.
Published: 2015-03-19.
Abstract
The aim of this study was to evaluate the predatory behavior in vitro of the mite Lasioseius penicilliger on 3 nematode species: Teladorsagia circumcincta (L3) (a sheep-parasitic nematode), Meloidogyne sp. (J2) (a plant-parasitic nematode), and on various developmental stages of Caenorhabditis elegans (a free-living nematode). The coincubation of mites and nematodes was individually assessed in 2% water agar placed in plastic Petri dishes (2 cm x 1 cm diameter). One thousand nematodes of a species and 5 mites were placed into each plate (10 replicates) and incubated for 5 days at room temperature (18-25ºC). L. penicilliger showed predatory behavior against the 3 assessed nematode species. The percentages of predatory activity recorded were 95.1, 80.5 and 79.3 against Meloidogyne sp., C. elegans, and T. circumcincta, respectively (P ≤ 0.05). These results suggest that L. penicilliger has important potential as a biological control agent of parasitic nematodes.
Keywords: Predatory mite; Parasitic nematodes; Neotropical.
Introduction
The parasitic nematodes are responsible for severe diseases to plants and animals and are a major concern to the livestock and agricultural industries (Fitzpatrick, 2013). The nematode Teladorsagia circumcincta, for example, is one of the most economically important parasitic nematodes of sheep in cool temperate regions (Gossner et al., 2012). For this reason, farmers use different chemicals for controlling nematode parasites, although the indiscriminate use of drugs against parasitic nematodes and the ensuing development and growth of drug resistance in the parasites have led researchers worldwide to search for new control strategies (Kaplan, 2004; Davies and Spiegel, 2011; Good et al., 2012; Torres-Acosta et al., 2012b).
Therefore, the need has arisen for alternatives to chemical control such as biological control, which is a relevant option (Sayre and Walter, 1991; Timper, 2011). With respect to the economic importance of parasitic nematodes of ruminants, the need for molecular tools to specifically diagnose nematode infections for refined investigations of parasite epidemiology and drug resistance detection in combination with conventional methods must be emphasized (Roeber et al., 2013). Additionally, the production of different crops is affected by a variety of pathogenic organisms that seriously affect the production, development and plant vigor (Back et al., 2002). Among these agents are the phytonematodes, which cause diverse symptomatology depending on the genus and species of nematodes, and that may affect different parts of the plant. The degree of pathogenicity depends on the aggressiveness of the strain as well as the anatomical and physiological adaptations of each plant to parasitism (Dutta et al., 2011). In this context, Meloidogyne spp., which belongs to the group of gall-forming nematodes, is considered one of the most important pests in various crops, primarily in tropical and subtropical countries where it is widely distributed (Luc et al., 2005), causing severe annual economic losses estimated at $125 million globally (Hodda and Cook, 2009; Safdar and McKenry, 2012; Sikora and Fernandez, 2005). As in the case of ruminant parasitic nematodes, alternative control methods, such as biological control approaches, are urgently needed to alleviate the huge economic burden that these parasitic nematodes cause to the farming industry (Van der Putten et al., 2006).
Nematodes in the soil have several natural enemies, such as viruses, protozoa (Bjornlund and Ronn, 2008), flatworms, insects, tardigrades (Sayre and Walter, 1991), nematode "predators" of other nematodes (Bilgrami, 2008), bacteria, nematophagous fungi (Mendoza de Gives and Torres-Acosta, 2012) and mites (Aguilar-Marcelino et al., 2014).
Mesostigmata mites of the genus Lasioseius (Berlese, 1916) are distributed worldwide and belong to the Family Ascidae (Berlese). The species of this genus are considered predators and can be found on a variety of substrates, such as soil, litter and in association with insects and vertebrates (Walter and Lindquist, 1997).
In particular, the species L. penicilliger has advantages as a potential biological control agent due to characteristics such as its short life cycle, and parthenogenetic reproduction, which allows for medium-term population increases. It is important to note that the L. penicilliger used in the present study has been maintained in the laboratory using nematodes as food for 5 years. This may be important for selecting mites with preferences for parasitic nematodes as food. There have been few studies on the use of mites as control agents of parasitic nematodes. The aim of the present investigation, therefore, was to evaluate the in vitro predatory activity of L. penicilliger on T. circumcincta (L3), Meloidogyne sp. (J2) and C. elegans to test the hypothesis that this mite feeds differently on nematodes depending on their size and on the presence or absence of an outer cuticle.
Material and methods
The origin of biological material used was as follows:
Mite
Lasioseius penicilliger (Arachnida: Mesostigmata) was isolated from soil samples in Morelos, Mexico, in 2009 and identified according to Hughes (1976). Since then, the mite strain has been kept in the laboratory of Helminthology of CENID-PAVET by culturing in Petri dishes (2 cm diameter and 1 cm high) containing 2% water agar at room temperature, (27 ± 2ºC) under dark conditions (Bilgrami, 1994). Panagrellus redivivus (Nematoda) were used as food, once a week, for the mites in the culture dishes. Adult mites, both males and females, were randomly used.
Nematodes
• Teladorsagia circumcincta. This nematode species was collected from a naturally parasitized deer at Guerrero state, Mexico, in 2011 (Liébano-Hernández, unpublished) and was identified according to its morphological characteristics (Indre et al., 2011; Van Wyk et al., 2013). It has since been maintained, as a pure isolate, at the CENID-PAVET by continuous passages into susceptible young sheep.
• Meloidogyne spp. This nematode was isolated from infected tomato plants (Lycopersicon esculentum Mill) from Jojutla Municipality, Morelos state, Mexico. This strain was cultured under greenhouse conditions at Jiutepec, Morelos state, Mexico, and was maintained by successive passages in tomato plants under controlled conditions.
• Caenorhabditis elegans. The strain N2, variety Bristol, was used; this nematode was cultured in Petri dishes containing NGM (nematode growth medium). As a first step, a wild Escherichia coli commercial strain (ER2738, New England The Biolabs) was grown in NGM for 2 h at 37ºC. An abundant nematode population was achieved by transferring them to new Petri dishes containing bacteria growing on NGM for 3 days (Carvalho et al., 2014).
Experimental design
The predatory capacity of mites against nematodes was assessed using plastic Petri dishes (2 cm diameter x 1 cm high). Each Petri dish was considered as one experimental unit, which contained 2% bacteriologic agar in water (WAPD). During the experiment the dishes were kept at room temperature (18-25ºC).
The experiment consisted of placing 5 L. penicilliger adult and 1000 nematode larvae in each WAPD and 10 replicates of each treatment were made. The treatments were established as follows: S1: T. circumcincta (L3); S2: Meloidogyne sp. (J2); S3: C. elegans. Each WAPD was incubated for 5 days. At the end of this period, the mites were manually separated from every WAPD and the WAPD was washed with running water to collect the larvae. After 3 washings, nematodes of each series were recovered by the Baermann funnel technique after 12 h (Thienpont et al., 1986).
Nematodes were then counted by placing ten 5 μL aliquots on a glass slide and examining them under an optical microscope (10X). After data recording, survival rate and the predation percentage of L. penicilliger on each nematode species was estimated as follows:
Survival rate = number of recovered larvae / 1000

Where mnn = mean of the number of nematodes
Statistical analysis
The data on survival rate were normalized using the arcsine square root transformation and analyzed as a completely randomized design in a factorial arrangement of treatments (3 nematode species x 2 levels of mites – absence/presence). Means were compared using the Tukey test (SAS, 1998). A P ≤ 0.05 value was considered as significant.
Results
After 5 days of nematode-mite coincubation, the numbers of recovered larvae (mean ± standard deviation) in control (C) and treated (T) groups were 792 ± 233 (C) and 164 ± 262.9 (T) for T. circumcincta; 415 ± 117.9 (C) and 20 ± 34 (T) for Meloidogyne sp., and 335 ± 166.7 (C) and 65 ± 66 (T) for C. elegans. The predation percentage of L. penicilliger was 79.3% on T. circumcincta, 95.1% on Meloidogyne sp., and 80.5% on C. elegans (Fig. 1). Notably, none of the mites died during the experiment (data not shown).
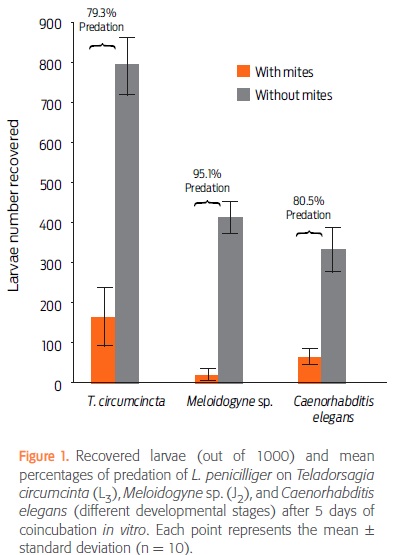
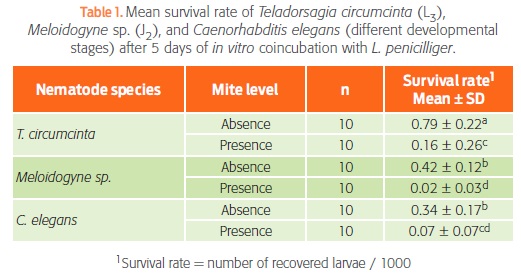
Table 1 shows the results from the analysis of variance. As expected, survival rate was always lowest in the presence of mites (P < 0.05). In the absence of mites, T. circumcincta had a higher survival rate than the other 2 nematodes, possibly because Meloidogyne sp. and C. elegans were completing their cycles and had no food during the 5 days of testing, whereas T. circumcincta was in its infective stage and did not need food. In the presence of L. penicilliger, the survival rate of C. elegans was similar to that of Meloidogyne sp. and T. circumcincta (P > 0.05), but the survival rate of Meloidogyne sp. was significantly lower than that of T. circumcincta (P < 0.05).
Discussion
Mites are being considered as promising bio-control agents of a number of agriculture pests (Chen et al., 2013). The results of this study support this strategy on the basis that L. penicilliger was able to prey on the 3 different assessed nematodes, regardless of their taxonomic origin.
The survival rate of Meloidogyne sp. (J2), however, was lower than that of T. circumcincta (L3), suggesting a selective predatory behavior of mites against different nematode taxons that may be related to differences between the surface structure of plant parasitic nematodes (Meloidogyne sp.) and animal parasitic nematodes (T. circumcincta) (Gravato-Nobre and Evans, 1998). Raleigh et al (1996), for example, found the presence of a sheath in ruminant parasitic nematode larvae, which acts as a protective coat. Alternately, other members of genus Lasioseius spp., e.g., L. subterraneus, have shown an enormous voracious activity against root-knot nematodes (Walter et al., 1993). However, there is thus far very limited information about the feeding habits of L. penicilliger as a predatory mite of animal parasitic nematodes.
This species has recently displayed a lethal potential in vitro activity against H. contortus infective larvae (Aguilar-Marcelino et al., 2014). Some information about the predatory activity of other Lasioseius species has been recorded against economically important plant-parasitic nematodes. For instance, L. scapulatus showed 99% in vitro predatory activity against Aphelenchus avenae (Imbriani and Mankau, 1983). With respect to the predatory activity of L. penicilliger against animal parasitic nematodes, a recent in vitro study showed 80% predation of this mite against Haemonchus contortus infective larvae (Aguilar-Marcelino et al., 2014). Such observations suggest that perhaps L. penicilliger acts similarly against other members of the Trichostongylidae family. These results also indicate a high predatory activity of L. penicilliger on the 3 assessed nematode species.
The fact that 2 species of nematodes assessed in the present study, T. circumcincta and Meloidogyne sp., are important pathogens for ruminants and plants may have important implications on further studies searching for a possible application of biologic control agents against both animal and plant nematode plagues.
In this regard, it is important to emphasize that the natural habitat of infective larvae of T. circumcincta and the other members of the group of ruminant parasitic nematodes is within fecal matter. An increase in predacious mite populations has been achieved with organic manure, in studies in which predacious mites were used for the control of citrus nematodes, (El-Banhawy et al., 1997). Therefore, perhaps one possible application of predacious mites, ie. L. penicilliger, for controlling ruminant parasitic nematodes may be through their use under field conditions on feces. This is, however, currently only speculation because the results were obtained in vitro and should be taken with caution. On the other hand, the fact that L. penicilliger acted against C. elegans (a free-living nematode) could be an undesirable feature. Much work is thus needed to further establish the potential of mites as biocontrol agents, considering that L. penicilliger is able to feed on both animal and plant parasitic nematodes.
Conclusions
The present research revealed important in vitro predatory activity by the mite L. penicilliger against T. circumcincta infective larvae. At this time, it is unclear how biological control using mites could reduce the parasitic larvae population in the field.
Funding
This study was financed by a grant from SAGARPA-CONACYT (Project No. 11990/2005).
Acknowledgements
The authors wish to acknowledge Dr. Enrique Liébano Hernández's active participation in this research before he passed away.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
Noemí García Ortiz and María Eugenia López Arellano: Conducted the experiment and critically reviewed and approved the manuscript for publication.
Liliana Aguilar Marcelino: Designed the experiment and critically reviewed and approved the manuscript for publication.
Pedro Mendoza de Gives: Analyzed the data and critically reviewed and approved the manuscript for publication.
Carlos Ramón Bautista Garfias: Analyzed the data and drafted and approved the manuscript for publication.
Roberto González Garduño: Reviewed the statistical analysis and drafted and approved the manuscript for publication.
1) Aguilar-Marcelino L, Quintero-Martínez MT, Mendoza de Gives PME, López-Arellano ME, Liébano-Hernández E, Torres-Hernández G, González-Camacho JM, Cid del Prado I. 2014. Evaluation of predation of the mite Lasioseius penicilliger (Arachnida: Mesostigmata) on Haemonchus contortus and bacteria-feeding nematodes. Journal of Helminthology, 88:20-23. [ Links ]
2) Back MA, Haydock PPJ, Jenkinson P. 2002. Disease complexes involving plant parasitic nematodes and soil borne pathogens. Plant Pathology, 51:683-697. [ Links ]
3) Bilgrami AL. 1994. Predatory behavior of a nematode feeding mite Tyrophagous putrescentiae (Sarcoptiformes: Acaridae). Fundamental and Applied Nematology, 17:293-297. [ Links ]
4) Bilgrami AL. 2008. Biological control potentials of predatory nematodes. In: A. Ciancio, KG Mukerji (editors) Integrated Management and Biocontrol of Vegetable and Grain Crops Nematodes, Springer. [ Links ]
5) Bjornlund L, Ronn R. 2008. "David and Goliath" of the soil food web-Flagellates that kill nematodes. Soil Biology and Biochemistry, 40:2032-2039. [ Links ]
6) Carvalho S, Phillips CP, Teotónio H. 2014. Hermaphrodite life history and the maintenance of partial selfing in experimental populations of Caenorhabditis elegans. Evolutionary Biology, 14:117. http://www.biomedcentral.com/1471-2148/14/117. [ Links ]
7) Chen YL, Xu CL, Xu XN, Xie H, Zhang BX, Qin HG, Zhou WQ, Li DS. 2013. Evaluation of predation abilities of Blattisociusdolichus (Acari: Blattisociidae) on a plant-parasitic nematode, Radopholus similis (Tilenchida: Pratylenchidae). Experimental Applied Acarology, 60:289-298. [ Links ]
8) Davies K, Spiegel Y (eds.) 2011. Biological control of plant-parasitic nematodes: building coherence between microbial ecology and molecular mechanisms. Springer. [ Links ]
9) Dutta TK, Stephen JP, Kerry BR, Gaur HS, Curtis RHC. 2011. Comparison of host recognition, invasion, development and reproduction of Meloidogyne graminicola and M. incognita on rice and tomato. Nematology, 13:509-520. [ Links ]
10) El-Banhawy EM, Osman HA, El-Sawaf BM, Afia SI. 1997. Interaction of soil predacious mites and citrus nematodes (parasitic and saprophytic), in citrus orchard under different regime of fertilizers. Effect on the population densities and citrus yield. Anzeiger für Schädlingskunde, Pflanzenschtuz, Umweltschutz, 70:20-23. [ Links ]
11) Fitzpatrick JL. 2013. Global food security: the impact of veterinary parasites and parasitologists. Veterinary Parasitology, 195:233-248. [ Links ]
12) Good B, Hanrahan JP, Theodorus de Waal D, Patten T, Kinsella A, Oliver LC. 2012. Anthelmintic-resistant nematodes in Irish commercial sheep flocks-the state of play. Irish Veterinary Journal, 65(1):21. DOI: 10.1186/2046-0481-65-21. http://www.irishvetjournal.org/content/65/1/21. [ Links ]
13) Gossner AG, Venturina VM, Shaw DJ, Pemberton JM, Hopkins J. 2012. Relationship between susceptibility of blackface sheep to Teladorsagia circumcincta infection and an inflammatory mucosa T cell response. Veterinary Research, 43:26 http://www.veterinaryresearch.org/content/43/1/26. [ Links ]
14) Gravato-Nobre MJ, Evans K. 1998. Plant and nematode surfaces: their structure and importance in host-parasite interactions. Nematologica, 44:103-124. [ Links ]
15) Hodda M, Cook DC. 2009. Economic impact unrestricted spread cyst trophic composition. Phytopathology, 99:1987-1393. [ Links ]
16) Hughes AMH. 1976. The mites of stored food and houses. Technical bulletin 9. London, England: Her Majesty's Stationery Office, Ministry of Agriculture, Fisheries and Food. [ Links ]
17) Imbriani IY, Mankau R. 1983. Studies on Lasioseius scapulatus, a mesostigmatid mite predaceous on nematodes. Journal of Nematology, 15:523-528. [ Links ]
18) Indre D, Balint A, Hotea I, Sorescu D, Indre A, Darabus G. 2011. Trichostongyles species and other gastrointestinal nematodes identified in sheep from Timis County. Bulletin UASVM, Veterinary Medicine, 68:171-178. http://journals.usamvcluj.ro/index.php/veterinary/article/viewFile/6897/6159. [ Links ]
19) Kaplan RM. 2004. Drug resistance in nematodes of veterinary importance: a status report. Trends in Parasitology, 20:477-481. [ Links ]
20) Luc M, Sikora RA, Bridge J. 2005. Plant parasitic nematodes in subtropical and tropical agriculture. 2nd ed. London, UK: CAB International. pp. 1-10. [ Links ]
21) Mendoza de Gives P, Torres Acosta JFJ. 2012. Biotechnological use of fungi in the control of ruminant parasitic nematodes. En: Paz Silva A, Sol M (eds.) Fungi: Types Environmental Impact and Role in Disease. : Nova Science Publisher, Inc. pp. 389-408. [ Links ]
22) Raleigh MG, Brandon MR, Meeusen E. 1996. Stage-specific expression of surface molecules by the larval stages of Haemonchus contortus. Parasite Immunology, 18:125-132. [ Links ]
23) Roeber F, Jex AR, Gasser RB. 2013. Impact of gastrointestinal parasitic nematodes of sheep, and the role of advanced molecular tools for exploring epidemiology and drug resistance-an Australian perspective. Parasites & Vectors, 6:153. DOI: 10.1186/1756-3305-6-153 http://www.parasitesandvectors.com/content/6/1/153. [ Links ]
24) Safdar AA, McKenry MV. 2012. Incidence and population density of plant-parasite nematodes infecting vegetable crops and associated yield losses in Punjab Pakistan. Pakistan Journal of Zoology, 44:327-333. [ Links ]
25) SAS Institute. 1998. Statistical software Ver. 6.03. Cary, North Carolina, USA. [ Links ]
26) Sayre RM, Walter DE. 1991. Factors affecting the efficacy on natural enemies of nematodes. Annual Review Phytopathology, 29:149-166. [ Links ]
27) Sikora RA, Fernandez E. 2005. Nematode parasites of vegetables. En: Luc M, Sikora RA, Bridge J (eds.) Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 2nd ed. Wallingford, UK: CAB International. pp. 319-392. [ Links ]
28) Timper P. 2011. Utilization of biological control for managing plant-parasitic nematode. En: Biological Control of Plant-Parasitic Nematodes. Hokkanan HMT (ed.) Springer Dordrecht Heidelberg London New York. pp. 259-260. [ Links ]
29) Thienpont D, Rochette F, Vanparijs OFJ. 1986. Diagnosing helminthiasis by coprological examination. 2nd ed. Beerse, Belgium: Janssen Research Foundation. pp. 35-36. [ Links ]
30) Torres-Acosta JFJ, Molento M, Mendoza-de-Gives P. 2012a. Research and implementation of novel approaches for the control of nematode parasites in Latin America and the Caribbean: is there sufficient incentive for a greater extension effort? Veterinary Parasitology, 186:132-42. [ Links ]
31) Torres-Acosta JFJ, Mendoza-de-Gives P, Aguilar-Caballero AJ, Cuéllar-Ordaz JA. 2012b. Anthelmintic resistance in sheep farms: update of the situation in the American continent. Veterinary Parasitology, 189:89-96. [ Links ]
32) Van der Putten WH, Cook R, Costa S, Davies KG, Fargette M, et al. 2006. Nematode interactions in nature: models for sustainable control of nematode pests of crop plants? Advances in Agronomy, 89:227-260. [ Links ]
33) Van Wyk JA, Mayhew E. 2013. Morphological identification of parasitic nematode infective larvae of small ruminants and cattle: a practical lab guide. Onderstepoort Journal of Veterinary Research, 80(1). DOI: 10.4102/ojvr.v80i1.539 http://www.ojvr.org/index.php/ojvr/article/view/539. [ Links ]
34) Walter DE, Kaplan DT, Davis EL. 1993. Colonization of greenhouse nematode cultures by nemathophagous mites and fungi. Supplement to Journal of Nematology, 25(4S): 789-794. [ Links ]
35) Walter DE, Lindquist EE. 1997. Australian species of Lasioseius (Acari: Mesostigmata: Ascidae): the porulosus-group and other species from rainforest canopies. Invertebrate taxonomy, 11(4):525-547. [ Links ]
Note This article can be read in its full version in the following page: http://www.revistas.unam.mx/index.php/Veterinaria-Mexico Mailing address:
Mailing address:
Liliana Aguilar Marcelino
E-mail: aguilar.liliana@inifap.gob.mx











 text in
text in 


