Introduction
Parasites from the genus Sarcocystis are protozoans of the phylum Apicomplexa. These parasites affect mammals, birds, reptiles, amphibians, and fish (Munday et al., 1979; Bolon et al., 1989; Hillyer et al., 1991; Dubey et al., 2001a; Dubey et al., 2003). Sarcocystis falcatula is the most prevalent species in birds, which serve as intermediate hosts, with opossums (Didelphis virginiana and Didelphis albiventris) serving as the definitive hosts (Box and Duszynski, 1978; Box and Smith, 1982; Dubey et al., 2000; Dubey et al., 2001b). Until 1995, the opossum was thought to be the definitive host of only S. falcatula; however, the opossum is known also to be the definitive host of S. neurona, S. speeri (Fenger et al., 1997; Dubey et al., 1998; Dubey et al., 1999) and S. lindsayi (Dubey et al., 2001c). Mansfield et al. (2008) reported S. neurona in brown-headed cowbirds. Dame et al. (1995) found a strong similarity between S. neurona and S. falcatula based on an analysis of the 18S rRNA gene and, thus, suggested that these are actually the same species. Subsequent biological studies, however, confirmed that these are indeed two different species (Marsh et al., 1997a; Dubey and Lindsay, 1998). Genetic typing by various methods has established that S. neurona and S. falcatula are distinct species. Polymerase chain reaction (PCR) primers targeting the SSU rRNA gene were developed that distinguished S. neurona from the Sarcocystis found in skunks, raccoons, hawks, coyotes and cats (Fenger et al., 1995). In another study, sequencing of the internal transcribed spacer 1(ITS-1) region of the rRNA gene showed that S. falcatula may be composed of a heterogeneous population and that the ITS-1 region can be used to distinguish S. neurona from S. falcatula (Marsh et al., 1999). Tanhauser et al. (1999) used ITS-1 to design specific primers (25/396) and restriction enzymes (Hinf I and Hind III) to perform PCR-restriction fragment length polymorphism (RFLP) analysis to differentiate S. neurona and S. falcatula. Likewise, Elsheikha et al. (2005) have used DNA markers 25/396 of ITS-1 for phylogenetic studies of S. neurona in the United States. Infections caused by S. neurona have been reported in a variety of species, such as cats (Butcher et al., 2002), raccoons (Dubey et al., 2001d), armadillos (Cheadle et al., 2001a), skunks (Cheadle et al., 2001b), seals (Miller et al., 2001), and sea otters (Dubey et al., 2003). In addition, S. neurona is an important cause of neurological problems in horses in the United States (Dubey et al., 1991; MacKay et al., 2000), although horses appear to be aberrant hosts (Dubey 2001b). Interestingly, schizonts have been observed in the brain and spinal cord and mature cysts have been observed in the skeletal muscle in a 4-year-old mare, suggesting that horses are intermediate hosts (Mullaney et al., 2005). In 2008, the presence of parasitic cysts of S. neurona in the skeletal muscles of brown-headed cowbirds (Molothrus ater) was documented, indicating that these birds may be intermediate hosts (Mansfield et al., 2008). In Mexico there are no data available on the presence of S. neurona in birds, but Yeargan et al., in 2013, found a seroprevalence of 48.5% of S.neurona in horses in northern Mexico.
The objective of this study was to describe the morphological and ultrastructural characteristics, the PCR-RFLP results, the sequences and the phylogenetic analysis of a specific fragment of internal transcribed spacer 1 (ITS-1), which was amplified using the 25/396 primers, of the Sarcocystis sp. parasites identified in the muscles of wild great-tailed grackles, bronzed cowbirds, and stripe-headed sparrows in Mexico that may be S. neurona.
Materials and methods
Fifteen wild birds (7 great-tailed grackles [Quiscalus mexicanus], 6 bronzed cowbirds [Molothrus aeneus], and 2 stripe-headed sparrows [Aimophila ruficauda]) found dead with suspected poisoning in the State of Morelos, Mexico, were submitted to the Diagnostic Laboratory and Research on Diseases of Birds of the Department of Medicine of Birds of the Faculty of Veterinary Medicine of the National Autonomous University of México (UNAM). During necropsy, parasitic structures were found in of the muscle.
Necropsy and histopathology
All birds were submitted for necropsy, and samples were taken from muscle, fixed in 10% buffered formalin, processed by routine histological techniques, embedded in paraffin, cut into 4-µm sections, and stained with hematoxylin and eosin (H&E).
Electron microscopy
Sections (3 mm2) of striated skeletal muscle with parasitic cysts were taken from great-tailed grackles, fixed in 2.5% glutaraldehyde and, subsequently, post-fixed with 1% osmium tetroxide for 2 hours. Following washing with 0.1 M cacodylate buffer solution (pH 7.2), tissues were dehydrated with increasing concentrations of acetone. Sections were then embedded in epoxy resin (Epón 812, Electron Microscopy Sciences, Industry Road Hatfield, PA) and, finally, were polymerized at 60°C for 24 hours. Afterwards, 200-µm thick semifine cuts were made using an ultramicrotome, and samples were mounted on slides and contrasted with toluidine blue (Hayat, 2000). Fine cuts were made to achieve 60-µm samples, and the samples were then mounted on copper mesh grids, contrasted with uranyl acetate and lead citrate, and observed with an electron microscope (Zeiss EM-900, Zeiss, Oberkochen, Germany) at 80 kV.
DNA extraction
To isolate the parasite DNA, muscle samples with cysts of the parasites were macerated with a pestle and suspended in 20% phosphate buffered saline (PBS). Then, 250 µl of the suspension was mixed with 250 µl of lysis buffer solution (EDTA-SDS-Tris HCL; GibcoBRL, Grand Island, NY, USA) and 25 µl of proteinase K (20 mg/ml; Fermentas Inc., Glen Burnie, MD, USA). The samples were then incubated in a 37°C water bath for 2 hours followed by DNA purification with phenol-chloroform-isoamyl alcohol. The DNA was then precipitated with ethanol and hydrated.
PCR
The following primers were used for the detection of S. falcatula or S. neurona: 25 5'-CAC ACA AAA CAC CTG AAA GTC ACG TAC TT-3' and 396 5'-CCT GCC TCA CTT CGA CAC AT-3' (Sigma-Aldrich Corp., St. Louis, MO, USA). These primers amplify a 334-bp fragment of ITS-1 of the ribosomal DNA gene. The PCR conditions were as follows: 2 µl of Taq buffer, 0.4 µl of dNTPs (0.2 mM), 1 µl of primer (0.5 mM), 1.2 µl of MgCl2 (1.5 mM), 1 µl of Triton (0.10%), 1 µl of bovine serum albumin BSA) (0.015 mg/ml), 0.5 µl of Taq polymerase (2.5 U/µl; Fermentas Inc., Glen Burnie, MD, USA), 7.9 µl of diethylpyrocarbonate (DEPC) water and 5 µl (200 ng/µl) of DNA. The samples were amplified with a PCR Sprint Thermal Cycler (PCR Sprint Thermal Cycler, Thermo Fisher Scientific, Inc., Waltham, MA, USA) the following conditions: 1 cycle at 94°C for 5 minutes; 30 cycles at 94°C for 30 seconds, 62°C for 30 seconds and 72°C for 40 seconds; and 1 cycle at 72°C for 5 minutes. Then, 5 µl of each PCR reaction was electrophoresed on a 2% agarose gel, which was then stained with ethidium bromide and observed under ultraviolet light.
Sequencing
The approximately 334-bp PCR fragment that was observed on the agarose gel was cut out and purified with a QIAquick® Gel Extraction Kit (QIAGEN, Hilden, Germany) following the manufacturer's instructions. The purified fragment was visualized in a 1% agarose gel that was stained with ethidium bromide, along with the molecular weight marker GeneRuler (Fermentas Inc., Glen Burnie, MD, USA). PCR sequencing was performed with a BigDye® Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) following the manufacturer's instructions. The sequencing reactions were purified in CENTRI-SEP™ Spin Columns (CS-901, Princeton Separations, Adelphia, NJ, USA) following the manufacturer's instructions. Subsequently, the sequencing reactions were read with a 3130 Genetic Analyzer (Applied Biosystems Foster City, CA, USA). Sequencing of double-stranded DNA for the marker 25/396 was carried out to confirm the nucleotide sequence, and the primers 25/396 were used to obtain sense and antisense sequences.
Raw sequences (sense and antisense) were edited using MEGA version 4 software (Tamura et al., 2007). The consensus sequence yielded a file corresponding to each of the samples, and an alignment among the three sequences - the stripe-headed Sparrow (G1), the bronzed cowbird (T1), and the great-tailed grackle (Z5) - was carried out.
Phylogenetic analysis
Phylogenetic analysis was based on sequences of the 25/396 DNA marker obtained from G1, T1 and Z5 in addition to the sequences obtained from GenBank. Sequences were aligned, and a phylogenetic tree was constructed with the neighbor-joining (NJ) method using the Kimura 2-parameter (K-2P) model. Bootstrap support for the results of the NJ analysis was based on 1,000 replicate datasets generated from the original multiple sequence alignment.
RFLP
The approximately 334-bp PCR fragment that was observed on the agarose gel was digested with two restriction enzymes, Hinf I (Life Technologies, Carlsbad, CA, USA) and Hind III (Roche Applied Science, Indianapolis, IN, USA). The following RFLP conditions were used: 10 µl of PCR product, 3 µl of buffer, 1 µl of enzyme (Hinf I or Hind III), and 16 µl of DEPC water. The samples were incubated for 12 hours in a water bath at 37°C. Then, 15 µl of the reaction was electrophoresed on a 3% agarose gel that was stained with ethidium bromide and observed under ultraviolet light.
Results
The bodies of all birds were in good condition, and intramuscular parasitic cysts were found in the striated muscle of the breast, wings, and legs. These cysts were white in color, elongated, and undulating, and their dimensions ranged from 0.3 to 0.5 cm in length (Fig. 1). No pathological changes were evident in other organs.

Figure 1 Skeletal muscle of the thigh and leg of a great-tailed grackle with abundant parasitic structures of Sarcocystis (arrows). Cysts are white in color, undulating, and oriented along the longitudinal axis of the muscle.
Microscopic examination of the muscle tissue sections of the 15 birds showed multiple round (20 to 200 µm in diameter), elongated (100 to 4000 µm in length) cyst structures, each with a continuous thin wall (less than 2 µm) eccentrically compressing the myofibrils. Inside, the Sarcocystis parasites contained abundant bradyzoites that in some areas were separated by septa. In the central part of the larger cysts, an acellular, amorphous pale eosinophilic material that corresponded to degenerated protozoa was observed (Fig. 2). In 3 great-tailed grackles, discrete, multifocal, inflammatory cell aggregates composed of lymphocytes and plasma cells were observed around the cysts.

Figure 2 Histological section (H&E stain) of skeletal muscle of a greattailed grackle containing a mature Sarcocystis cyst within the myofibers and no inflammatory reaction. Cysts have an eosinophilic wall (arrow) and contain numerous viable bradyzoites (VB). Degenerate protozoa are observed in the central part (DP).
Electron microscopy
Striated skeletal muscle sections exhibited sarcoplasmic, thin-walled parasitic cysts consisting of a granular layer with villar protrusions containing numerous electron-dense microtubules that extended from the point to the base and beyond the underlying granular layer. The parasitic cysts showed some electrolucid merozoites beneath their walls, whereas toward the central portion, numerous mature bradyzoites were grouped together and separated by prolongations of the granular layer. Bradyzoites measured 4.1 µm in length and 1.6 µm in width and exhibited numerous micronemes in the anterior third of the conoid. The majority of nuclei are round with abundant electron-dense heterochromatin, which adhered to the internal nuclear sheath, and occasionally, a prominent nucleolus was noted (Fig. 3 y 4).

Figure 3 Transmission electron photograph of the skeletal striated muscle of a great-tailed grackle with sarcoplasm that exhibits a thin-walled, moderately electrolucid cyst (electrolucid basal substance, GI) with irregular projections (villar protrusions, V) and microtubules (*) in the interior. Inside of the cyst, numerous bradyzoites (Br) are shown. The parasitic membrane shows multiple protuberances (arrow). Adjacent to the cyst wall, distorted sarcomeres and numerous mitochondria (M) are observed. The nucleus is indicated (Nu). The sample was treated with a contrast technique that uses uranyl acetate and lead citrate. 7000x magnification.

Figure 4 Transmission electron micrograph of a bradyzoite in a longitudinal cut. Notice the conoid (C) and numerous micronemes (Mc). From the nucleus, round bodies of moderate electron density, which correspond to dense granules (Gd) intermingled with electrolucid granules are observed. The nucleus is located at the back of the conoid and exhibits abundant electron-dense heterochromatin (Nu). The sample was treated with a contrast technique that uses uranyl acetate and lead citrate. 12000x magnification.
PCR and sequencing
Following amplification with the 25/396 primer pair, 11 of the 15 skeletal muscle samples were positive (73%): 5 Mexican great-tailed grackles, 4 bronzed cowbirds, and 2 stripe-headed sparrows.
Raw sequences (sense and antisense) of one specimen from each species were included in the study. The sequences were edited, and the fragment lengths were 338 bp for G1, 338 bp for T1, and 323 bp for Z5. Alignment among the 3 sequences was carried out, and 100% similarity was observed (fig. 5). In fact, all 3 sequences were identical. As the sequencing results contained only 323 bp for the Gt5 sequence, the final 15 nucleotides from the other 2 samples were not aligned with this sequence.

Figure 5 Sequences of a specimen from each species included in the study: stripe-headed sparrow (G1), bronzed cowbird (T1), and great-tailed grackle (Z5). Sequences were edited, and the lengths of the sequences were 338 bp for G1, 338 bp for T1, and 323 bp for Z5. Alignment between the three sequences was carried out, and 100% similarity was observed.
Using the Basic Local Alignment Search Tool (BLAST) from GenBank, a comparison of the sequences obtained in this study and the sequences contained in the database was performed. A 96% similarity was found between the sequences here and the sequences corresponding to the following GenBank accession numbers: AY627839, AY627841, AY627842, AY627845, AY627848, AY 627850, AY627851, AY627859, AF093159 and AY627852. All sequences corresponded to the fragment of the genome of Sarcocystis neurona with the exception of AY627852, which corresponded to a fragment of the genome of Toxoplasma gondii and AF093159, which corresponded to a fragment of the genome of S. falcatula.
Phylogenetic and restriction fragment length polymorphism (RFLP) analysis
The sequences obtained in this study show a 100% homology between them. You may also notice that they are topologically more distant among the sequences used to construct the phylogenetic tree, not being related to any group previously reported. The phylogenetic distance between the sequences obtained in this work, the sequences of previously reported Sarcocystis neurona and the sequence from a sample collected in Brazil (Sarcocystis spp.) have a similar magnitude (Fig. 6).

Figure 6 Neighbor-joining phylogenetic tree constructed from a matrix generated by the Kimura 2-parameter method using 1000 bootstrap replicates. The obtained sequences are identical and are phylogenetically distant from any previously reported sequence.
The banding pattern following digestion of the 334-bp fragment from all positive cases using the Hinf I restriction enzyme consisted of three fragments of 140, 108, and 62 bp. Digestion with the Hind III restriction enzyme did not affect the 334-bp product (Fig. 7).
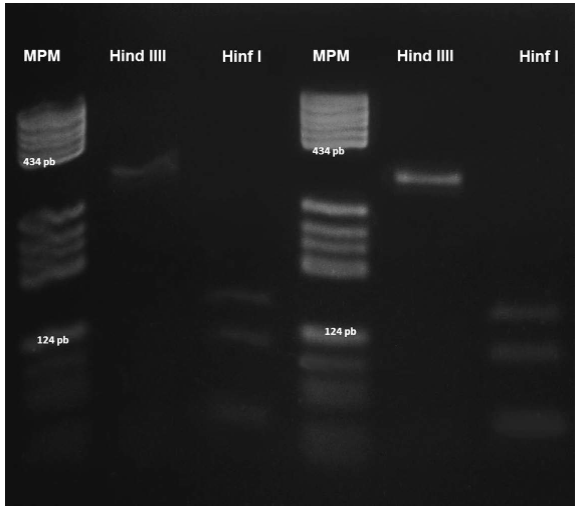
Figure 7 RFLP analysis of a Sarcocystis sp. parasite. A 334-bp fragment was amplified with the 25/396 primers. Lanes 1 and 4: PBR322/DNA/BsuRI (HaeIII) molecular weight marker; lanes 2 and 5: bronzed cowbird and great-tailed grackle samples treated with HindIII; lanes 3 and 6: bronzed cowbird and great-tailed grackle samples digested with HinfI.
Discussion
Sarcocystis were easily visualized in striated muscle that exhibited thin-walled parasitic cysts that measured between 0.3 and 0.5 cm in length. These observations differ from those reported by Mansfield et al (2008). In that study, the investigators analyzed 381 brown-headed cowbirds (Molothrus ater) from the United States and noted macroscopically visible Sarcocystis only in the legs. In addition, the histopathological examination revealed 2 types of cysts: thin-walled and thick walled. By electron microscopy, the thick-walled cysts were identified as S. falcatula, the thin-walled cysts were identified as S. neurona, and these findings were confirmed by PCR-RFLP. In the present study, macroscopic detection of Sarcocystis sp. parasites was common, possibly because the majority of the birds presented with chronic parasitic infection with mature Sarcocystis in the muscle fibers and degenerate bradyzoites in the interior, similar to the pattern in birds infected with S. falcatula. Presentation can be acute or chronic, depending on the affected bird. The acute presentation is generally observed in Old World psittacines and in pigeons and causes high mortality associated with pneumonia and encephalitis without the development of parasitic cysts. The chronic presentation occurs in American Passeriformes, which are intermediate hosts. No mortality is observed, and the disease is characterized by the formation of parasitic cysts in skeletal muscle, without a consequent inflammatory reaction (Villar et al., 2008). Histopathologic identification is subjective because S. neurona and S. falcatula have similar morphological characteristics, and literature data reports are inconclusive regarding the wall thickness of the parasitic cyst. Previously, however, Dubey et al. (2001e) reported the presence of a mature parasitic cyst with a 1 to 1.5-µm wall in the cerebellum of an ibis (Carphibis spinicollis), corresponding to Sarcocystis neurona-like parasites and similar to the protozoa described in this study. In this work, electron microscopy was only used to visualize Sarcocystis in great-tailed grackles, and the ultrastructural characteristics of the wall and bradyzoites coincide with the descriptions of S. neurona in bronzed cowbirds (Mansfield et al., 2008), horses (Mullaney et al., 2005), mice, and cell culture (Speer and Dubey, 2001). In these reports, the main characteristic for the ultrastructural identification of S. neurona is the accumulation of micronemes in the anterior third of the conoid end and a granular wall with numerous microtubules in villar protrusions. These studies suggest that the Sarcocystis parasites observed in pectoral muscles, legs, and wings of the three species of birds (wild great-tailed grackles, bronzed cowbirds, and stripe-headed sparrows) are similar to S. neurona.
Regarding the molecular identification of Sarcocystis in great-tailed grackles, bronzed cowbirds, and stripe-headed sparrows using PCR-RFLP (Tanhauser et al., 1999) with the 25/396 primers and Hinf I and Hind III restriction enzymes, a positive amplification product of 334 bp was observed. These products contained two Hinf I cutting sites in the same positions as the sequences of S. neurona. However, no recognition sites for Hind III were present in the 334-bp band. This finding is consistent with the banding pattern of S. neurona that was previously reported (Tanhauser et al., 1999; Mansfield et al., 2008; Mullaney et al., 2005; and Elsheikha et al., 2005).
Alignment among the 3 sequences was carried out, and 100% similarity was observed. A 96% similarity was found between the sequences studied and S. neurona. An additional cutting site that was not detected using PCR-RFLP was detected by sequencing. The generated fragments were approximately 140, 108, 62, and 24 bp. The additional cut by the HinfI enzyme may be due to a mutation or insertion of bases, due to independent genetic evolution during geographic isolation, as shown in the phylogenetic tree where the analyzed sequences are topologically separated between the US and South America. A similar result was observed by Elsheikha et al. (2005). These investigators examined the sequences of 25/396 fragments from 10 Sarcocystis neurona samples isolated and found mutations and base insertions, providing evidence for the presence of closely related genetic variants of S. neurona existing within the US and South America. Monteiro et al. (2013) suggest that it is possible that genetic groups of S. neurona and S. falcatula may exchange highly divergent alleles in sexual recombination.
However, because 25/396 marker sequence similarity is highly conserved among apicomplexan protozoans (Sarcocystis neurona, S. falcatula, Toxoplasma gondii, Neospora caninum), errors can occur in the interpretation of phylogenetic relationships. This finding emphasizes the importance of using more than just genetic or DNA markers for a robust phylogenetic analysis. In addition, these DNA studies should be supplemented with biological studies because, for example, mice are more susceptible to S. neurona but are resistant to S. falcatula (Dubey and Lindsay, 1998), while Australian parakeets are susceptible to S. falcatula and are not affected by S. neurona (Marsh et al., 1997b).
Conclusions
The morphological, ultrastructural and PCR-RFLP characteristics suggest that the parasitic cysts observed in the studied birds are S. neurona. However, in the phylogenetic tree, sequences are topologically distant from the published sequences of S. neurona from the United States and South America, which suggests that this may be a new subspecies of S. neurona.











 texto en
texto en 


