Introduction
Sea turtles belong to the class Reptilia and first appeared in fossil records approximately 200 million years ago (Pritchard, 1979). They belong to the order Testudines (Linnaeus, 1758), which includes the family Cheloniidae (Oppel, 1811). In total, there are 7 species of marine turtles (ITIS, 2000).
Loggerheads occur throughout a wide biogeographic distribution, inhabiting warm zones in the Pacific Ocean, the Atlantic Ocean, the Indian Ocean and the Mediterranean Sea (Spotila, 2004). Loggerheads are omnivorous feeders and their diets include items such as mollusks, crustaceans, porifera, corals, algae, as well as small vertebrates such as fish (Ernst and Lovich, 2009). In addition, loggerheads have benthic feeding and diving habits (Davenport and Clough, 1986; Ernst and Lovich, 2009); at an early age (when their weight is between 300 g and 400 g), loggerheads can swim to depths greater than 2 m and can be found feeding at depths up to 290 m as adults (Davenport and Clough, 1986).
In the loggerhead, as in other reptiles, the gastrointestinal tract (GIT) consists of the oral cavity, the esophagus, the stomach, the small and large intestines, and the cloaca (Wyneken, 2001). The small intestine (SI) covers approximately 50% of the total GIT (Thompson, 1980) and is divided into the duodenum, the jejunum, and the ileum. Macroscopically, the duodenum presents a honeycomb appearance (Wyneken, 2001). Chemical digestion starts in the stomach and continues in the SI, where enzymes that breakdown carbohydrates and proteins are added. The SI is specialized for the absorption of amino acids, carbohydrates, fatty acids, minerals (mainly calcium and phosphorus) and water (Wyneken, 2001).
To the best of our knowledge, neither histological nor histochemical descriptions have been performed on the SI of loggerhead hatchlings. In an attempt to yield information on this topic, we aimed to identify the microscopic morphological and histochemical features of the SI that possibly correlate with specific functions in loggerhead hatchlings. As the SI is a specialized organ related to digestion and nutrient absorption, we focused our research on its utility as a first approach to digestive physiology and pathology studies.
Materials and methods
Biological materials
The protocols for the use of the animals in this study were approved by the Institutional Animal Care and Use Committee (CICUAE) of the Facultad de Medicina Veterinaria y Zootecnia (FMVZ) of the Universidad Nacional Autónoma de México (UNAM) and the protocols of Parque Eco Arqueológico Xcaret.
Due to the endangered status of the loggerhead, this study was performed with dead animals in concordance with CICUAE recommendations. Fieldwork was conducted in collaboration with the Coordinación de Tortugas Marinas y Manatíes (Coordination of Sea Turtles and Manatees) of the Parque Eco Arqueológico Xcaret. This eco archaeological park is located in the state of Quintana Roo, Mexico (21°09'17'' N and 86°50'56'' W).
During fieldwork from June to July 2010, we performed several necropsies; however, only four individuals fulfilled the desired characteristics for this study (n=4): a time of death less than one hour prior to collection and no macroscopic alterations. The loggerhead turtles had been incubating in artificial nests inside Xcaret's aquarium and were found dead after hatching. No gross lesions were observed during the postmortem examination. During the necropsy, the two portions (cranial and caudal) were obtained by dividing the SI of each turtle exactly in half (n=8). This method was chosen due to the difficulty in macroscopically distinguishing the intestinal segments, as reported by Magalhaes (2012). The author defined the cranial SI portion as the duodenum and the caudal SI portion as the jejunum/ileum. The time lapse between death and fixation was approximately 30 minutes.
Histological processing
The samples were fixed in buffered Zinc-Formalin solution Z-Fix© (Anatech Ltd., MI, USA) for 48 hours after they were processed using paraffin embedding methods (Paraplast "R", McCormick Scientific© IL, USA). The samples were then processed with a histokinette (American Optical© T/P 8000) and sectioned using a microtome (Leica© RM 2125 RT) into 4 urn thick sections. The samples were attached to the slides, dewaxed in xylene (twice for 3 minutes). For rehydration, 100% and 95% ethanol solutions were used (twice each for 5 minutes). Then, the samples were washed in phosphate-buffered saline (PBS) for 5 minutes.
For the basic histological methods, Hematoxylin and Eosin (HE) and Gomori trichrome (GT) stains were performed (García del Moral, 1993). We also performed special staining methods (Pearse, 1960; García Del Moral, 1993, Prophet et al., 1995) such as the Periodic acid-Schiff reactive (PAS), Giemsa, toluidine blue (TB) at pH 4.5 and pH 3.5, alcian blue (AB) 8GX at pH 1.0 and 2.5, PAS/AB (AB 8GX pH 2.5/Periodic acid-Schiff reactive), colloidal iron, Perl's blue, methenamine silver (MS), Wilder's impregnation (WI) (Prophet et al., 1995), Grimelius and Masson-Fontana (M-F). For sample mounting, Permanent Mounting Medium was used (VectaMount® H-5000 CA, USA). The estimation of staining intensity was performed visually using the following parameters: (-) No staining, (+-) Very mild intensity, (+) Mild intensity, (++) Medium intensity, (+++) Strong intensity and (γ-M) gamma-metachromasia (García del Moral, 1993).
Image processing
Micrographs were taken using a Carl Zeiss® microscope (Primo Star®, Göttingen, Germany) connected to a Moticam 5® digital camera (Motic®, Xiamen, China). We used Motic Image Plus® software (Motic®, Version 2.0, Xiamen, China) to perform the measurements.
Nomenclature
We used nomenclature according to Nomina Anatómica Veterinaria Fifth edition (ICVGAN, 2012).
Results
Histology
The mucosa consisted of the epithelium, lamina propria and muscularis mucosae. The epithelium of the loggerhead SI was a pseudo-stratified columnar epithelium with interspersed goblet cells (GCs); the number was noticeably higher in the cranial portion (Figs. 1C, 1D, 1G, 1H), and the GCs had an elongated shape, typically in the shape of a chalice. By contrast, the GCs in the caudal portion were rounded and smaller sized.
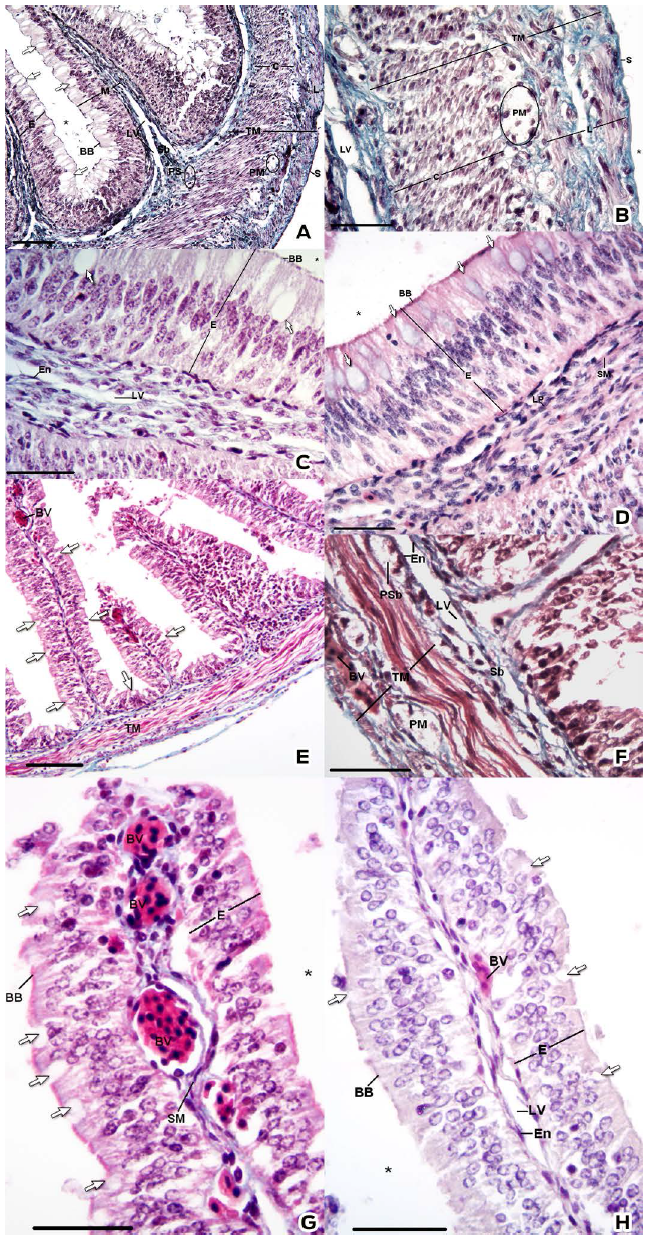
Figure 1 Histological characteristics of the small intestine. A, B, C, E, F and G: Gomori's trichrome stain. D and H: H&E stain. Epithelium (E), Mucosa (M), Submucosa (Sb), Smooth Muscle (SM), Tunica muscularis (TM), Serosa (S), Goblet cell (Arrow), Brush Border (BB), Blood Vessel (BV), Lymphatic Vessel (LV), Endothelium (En), Plexus mientericus (PM) and lumen (*). Bar: A and E) 100 µm. B, C, D, F, G) 30 µm.
The enterocytes (epithelial cells) had a brush border (Figs. 1C, 1D, 1G, 1H), and abundant blood vessels were observed in the lamina propria (Figure 1G). Below the basement membrane, the lamina propria was delimited by the muscularis mucosae that evaginated in the plicae and showed differences in length; it was longer in the cranial portion (Figs. 1A and 1E). These evaginations were confirmed by GT staining, which distinguishes the connective tissue from the smooth muscle (Figs. 1C and 1G).
Similarly, we observed different basement membrane strata beneath a simple squamous layer epithelium (endothelium), which formed ducts that appeared to be lymphatic vessels (Figs. 1C, 1F and 1H). In addition, we observed cells with large central nuclei and one or two nucleoli in the connective tissue beneath the lamina muscularis mucosae.
The tunica muscularis consists of two layers of smooth muscle: the stratum circulare and the stratum longitudinale (Figs. 1A, 1B, 1E and 1F); the stratum circulare is thicker than the stratum longitudinale. A group of cells was located between the circular and longitudinal muscle layers, and the cells were characterized by a large central nuclei and pale cytoplasm (Figs. 1B and 1F).
The tunica serosa was composed of loose connective tissue, and a mesothelium (Fig. 1B) covered the external surface of the gut.
Histochemistry
In the histochemical study, we observed important variations. The MPS histochemistry results are shown in Table 1.
Table 1 Summary of goblet cell histochemical results.
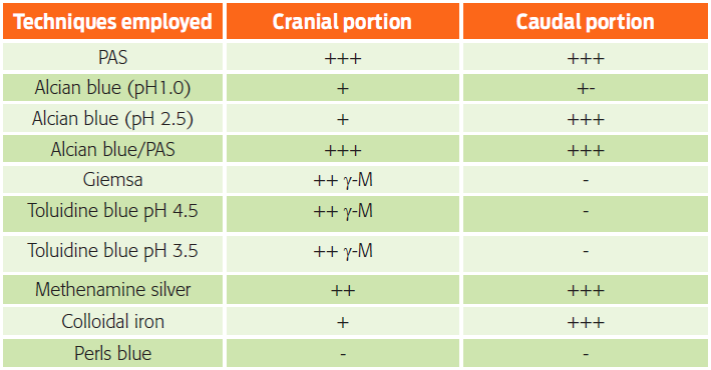
(-) no staining, (+-) very mild intensity, (+) mild intensity, (++) medium intensity, (+++) strong intensity and (γ-M) gamma-metachromasia.
The PAS stain, which detects periodate-reactive vicinal diols and glycogen (Mc Manus, 1948), confirmed that the epithelium was pseudostratified columnar (Figs. 2A and 3A). The apical cytoplasm of the GCs was PAS positive along the SI (Figs. 2A, 3A and Table 1). In the submucosa, the abundant basement membranes underlying the endothelium were stained specifically with PAS, MS and WI (Figs. 2A, 2E, 2F, 3A, 3E and 3F).
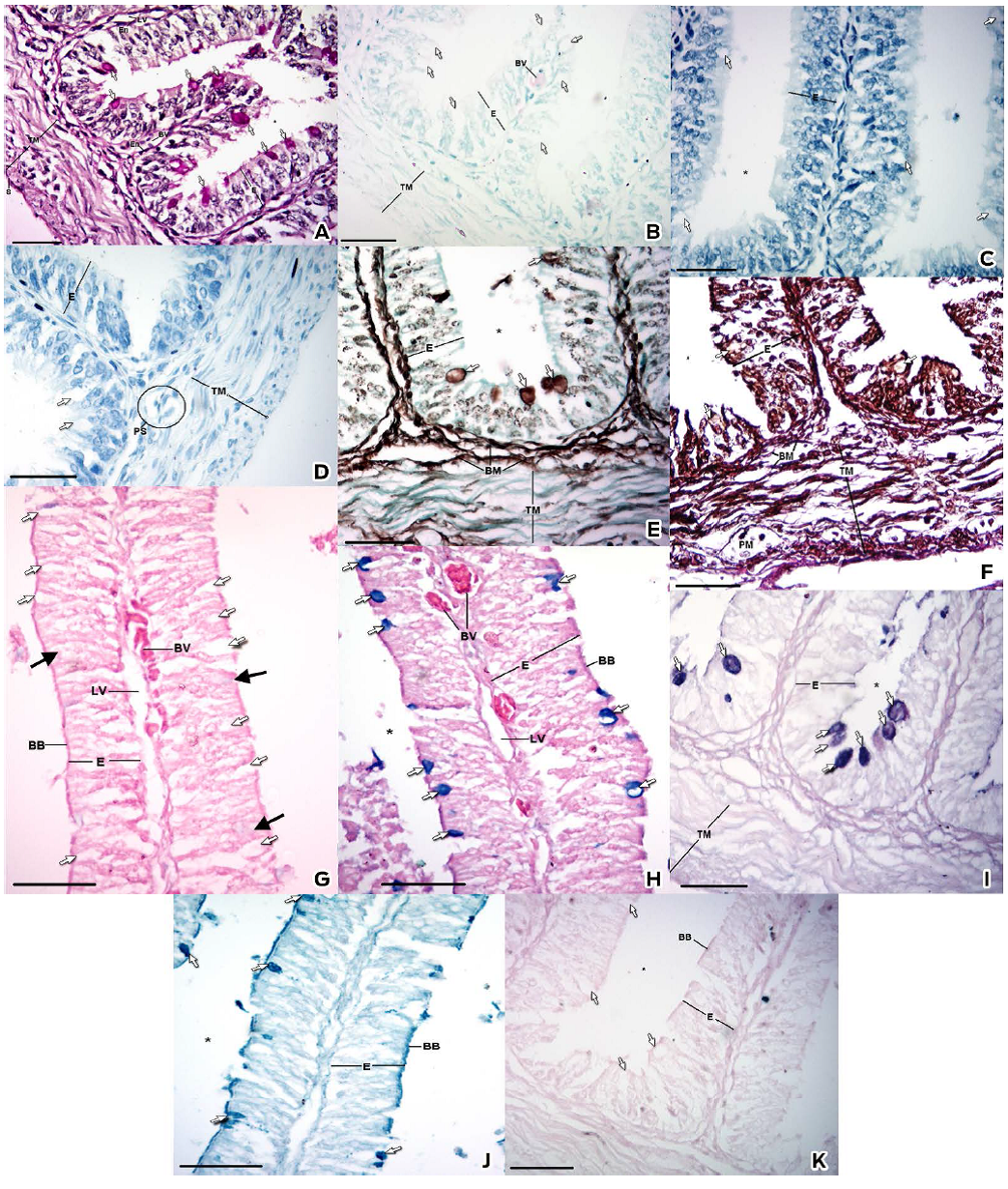
Figure 2 Histochemical characteristics of the cranial portion of the small intestine. A) PAS stain B) Giemsa stain C) Toluidine blue stain at pH 4.5. D) Toluidine blue stain at pH 3.5 E) Silver methenamine stain F) Wilder's impregnation G) Alcian blue pH 1.0 stain H) Alcian Blue pH 2.5 stain I) PAS/Alcian blue J) Colloidal Iron stain K) Perls Blue stain. Epithelium (E), Brush border (BB), Goblet Cell (Arrow), Basement Membrane (BM), Smooth Muscle (SM), Blood Vessel (BV), Lymphatic Vessel (LV), Endothelium (En), Tunica Muscularis (TM) and Lumen (*). Bar: 30 µm
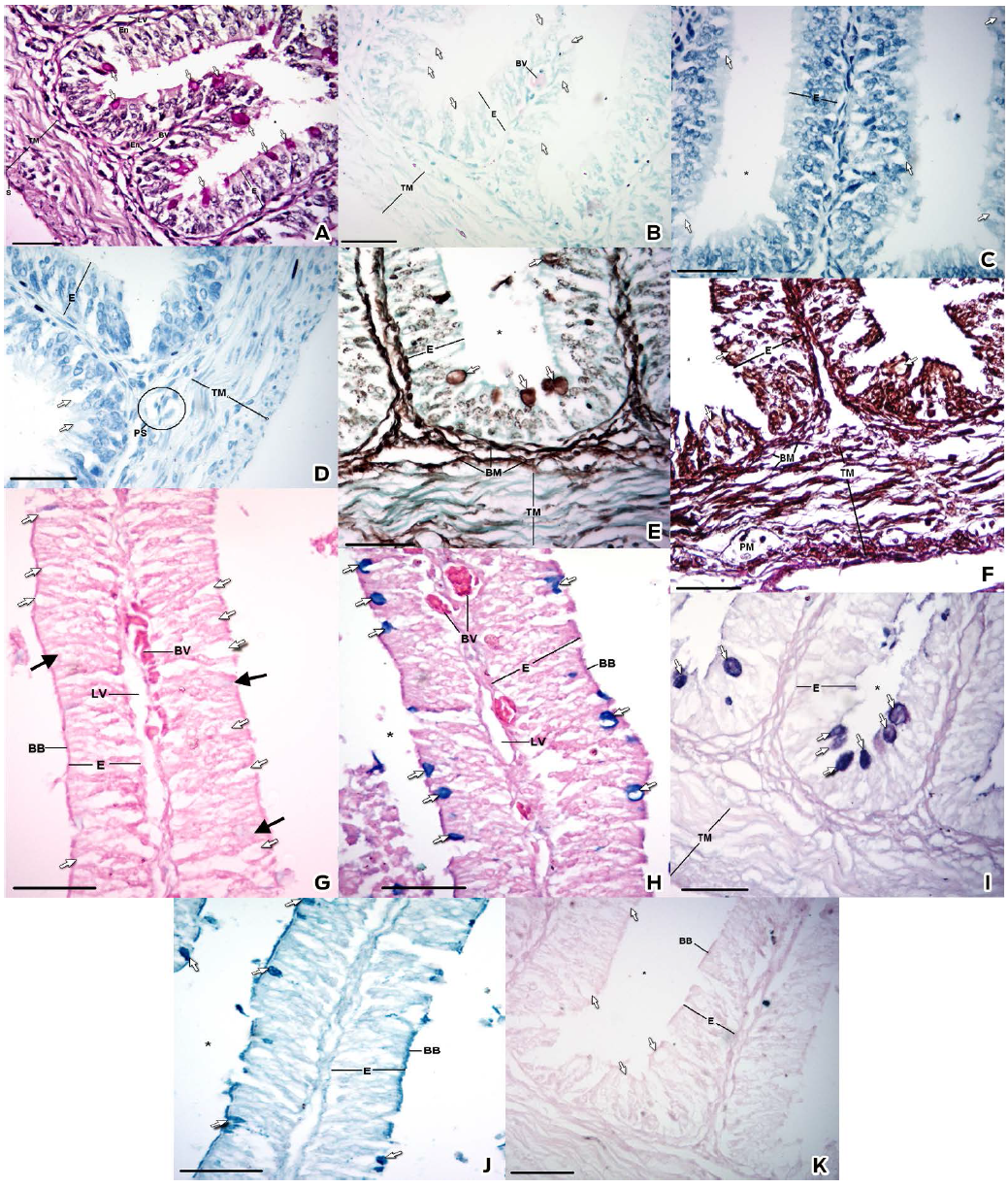
Figure 3 Histochemical characteristics of the caudal portion of the small intestine. A) PAS stain B) Giemsa stain C) Toluidine blue stain at pH 4.5 D) Toluidine blue stain at pH 3.5 E) Silver methenamine stain F) Wilder's impregnation G) Alcian blue pH 1.0 stain H) Alcian Blue pH 2.5 stain I) PAS/Alcian blue J) Colloidal Iron stain K) Perls Blue stain. Epithelium (E), Brush border (BB), Goblet Cell (Arrow), Goblet Cell stained with AB pH 1.0 (Black Arrow), Basement Membrane (BM), Smooth Muscle (SM), Plexus Submucosus (PS), Plexus Mientericus (PM), Blood Vessel (BV), Lymphatic Vessel (LV), Endothelium (En), Tunica Muscularis (TM) and Lumen (*). Bar: 30 µm.
We observed a metachromatic reaction with the Giemsa and the TB pH 4.5, corroborated by the TB pH 3.5 stains, which was resistant to the ethylic dehydration; therefore, the metachromatic reaction belongs to the gamma type due to the presence of sulfated esters (Pearse, 1960; Garcia del Moral, 1993). This reaction was found in the cranial portion (Figs. 2B, 2C, 2D and Table 1) but not in the caudal portion, where a negative staining was observed (Figs. 3B, 3C, 3D and Table 1).
The AB stain was used to determine the presence of acid mucopolysaccharides (AMPSs) (Mowry, 1956), AB at pH 1.0 for O-sulfated AMPS and AB at pH 2.5 for AMPS with O-sulfated esters and carboxylic groups (sialic acid or uronic acid) (Pearse, 1960; Garcia del Moral, 1993).
In the samples of the cranial portion stained with AB pH 1.0, the granules of the GCs were mildly stained (Fig. 2G). However, the granules of several GCs in the caudal portion showed a very mild staining, whereas others were negative (Fig. 3G).
In the samples of the cranial portion stained with AB pH 2.5, a tenuous reaction in the granules of the GCs was observed (Fig. 2H), whereas in the caudal portion the granules were intensely stained (Fig. 3H).
The PAS/AB technique distinguished between granules with neutral MPSs and AMPSs in the same cell. The technique detected AMPS with carboxyl groups and AMPSs with O-sulfate esters (turquoise), periodate-reactive vicinal diols (magenta), AMPSs with carboxyl groups and AMPSs with O-sulfate esters and periodate-reactive vicinal diols (purple) (Yamabayashi, 1987). The granules of the GCs in both intestinal portions showed an affinity to both colorants but with different distributions. In the cranial portion, the GCs showed specific granules; thus it was possible to distinguish an affinity for the PAS stain or the AB stain (Fig. 2I). By contrast, in the caudal half, a homogeneous stain was distinguished for mixes of colorants and was visualized as a purple tone (Fig. 3I).
Using the colloidal iron stain, MPSs with sialic acid (Rambourg, 1971; Pearse, 1960; García del Moral, 1993) were observed in the GCs along the SI (Figs. 2J, 3J and Table 1). The cranial portion showed a minor staining affinity (Fig. 2J) compared with the caudal portion (Fig. 3J). This result was corroborated by the Perls blue stain, which dismissed false positives; the GCs were not stained (García del Moral, 1993) (Figs. 2K, 3K and Table 1).
The MS stain and WI stain, which demonstrated reticular fibers from the lamina reticularis (Figs. 2E, 2F, 3E and 3F), were employed to determine the presence of basement membranes. The MS strain demonstrated, in addition to the presence of multiple basement membranes, an affinity in cytoplasmic vesicles of enterocytes and the secretory granules of the GCs in both intestinal portions, with greater staining intensity in the caudal portion (Figs. 2E and 3E).
The Grimelius stain, a silver impregnation technique, was used for the detection of cells with argyrophilic granules (Grimelius, 2004). Clustered cells were observed in the submucosa, in particular in the base of the plicae, and were isolated along the plicae adjacent to the epithelial basement membrane (Figs. 4A and 4E). These cells were more numerous in the cranial half, with approximately four or five cells per group, in contrast to the one or two cells per group observed in the caudal half (Figs. 4B and 4F).
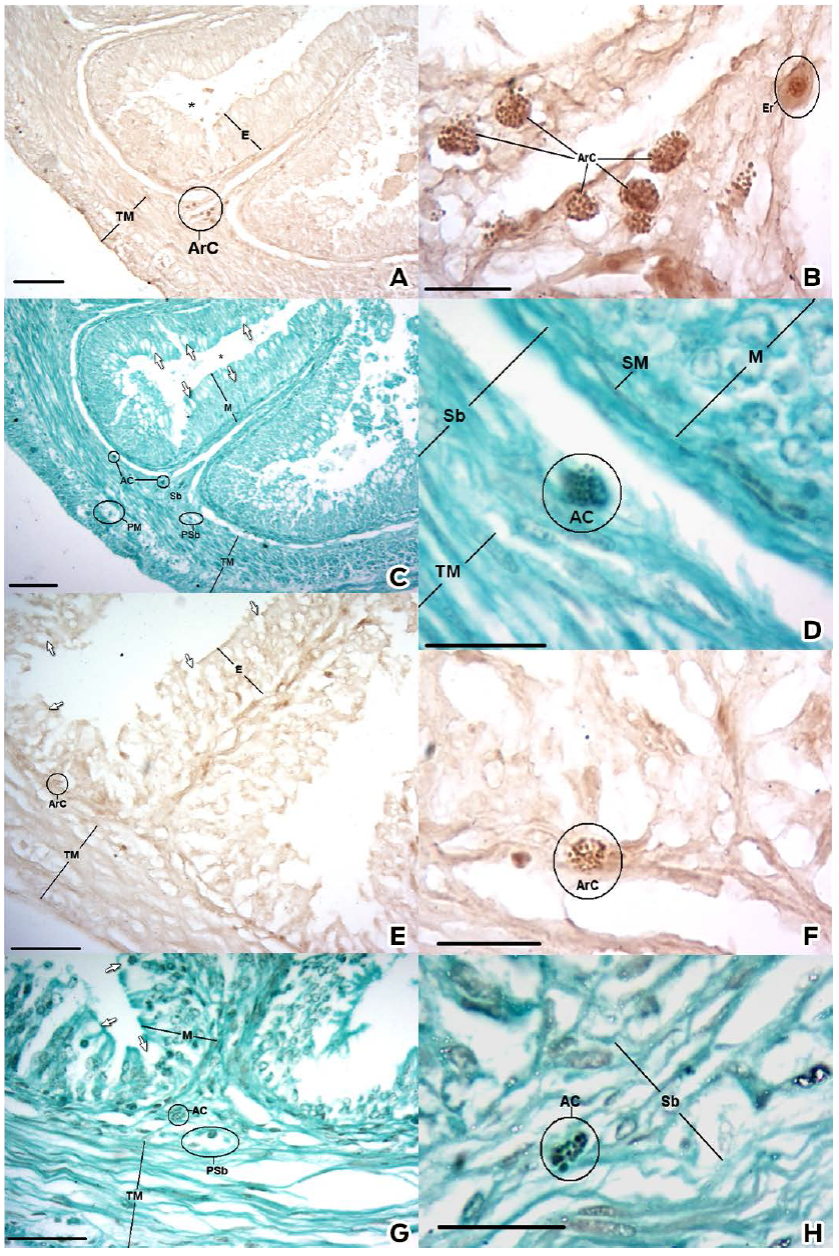
Figure 4 Histochemical results of the Grimelius and Masson-Fontana techniques. Cranial Portion: A and B) Grimelius stain, C and D) Masson-Fontana stain. Caudal Portion: E and F) Grimelius stain, G and H) Masson-Fontana stain. Epithelium (E), Mucosa (M), Brush Border (BB), Goblet Cell (Arrow), Argentaffin Cells (ArC), Argyrophilic Cells (AC), Erythrocyte (Er) Submucosa (Sb), Plexus Submucosus (Psb), Plexus Mientericus (PM), Blood Vessel (BV), Lymphatic Vessel (LV), Endothelium (En), Tunica Muscularis (TM) and Lumen (*). Bar: A and C) 100 μm. E and G) 30 μm. B, D, F, H) 20 μm.
The M-F stain was employed for the detection of molecules capable of reducing the silver based on the argentaffin reaction in the secretory granules (García del moral, 1993). Groups of cells were observed in a similar number, position and morphology as those found by the Grimelius stain (Figs 4C, 4D, 4G and 4H).
Lastly, the MPS distribution in the GCs along the SI presented the following: the cranial portion had neutral MPSs (Fig. 2A), sulfated AMPSs (Figs. 2B, 2C, 2D and 2G) and MPSs with sialic acid (Figs. 2J and 2K); the caudal portion had neutral MPSs (Fig. 3A) and MPSs with sialic acid (Figs. 3J and 3K).
Discussion
Histology
The brush border on the apical surface of the enterocytes (Figs. 1C, 1D, 1G, 1H) corresponds to the microscopic image of microvilli, which suggests that these cells have an increased surface area for absorption (Eichholz and Crane, 1965); however, this observation needs to be confirmed using electron microscopy.
Regarding the epithelium, none of the staining techniques (Figs. 1A, 2A and 3A) could demonstrate cell limits parallel to the basement membranes, which defines this epithelium as pseudostratified columnar; however, this should be confirmed by electron microscopy. Our finding is consistent with descriptions performed on the GIT of one amphibian: the caecilian Typhlonectes venezuelensis (Martínez et al., 2004). The authors proposed a hypothesis regarding the intestinal respiratory function based on the peculiarity of the pseudostratified epithelium. Certainly, it is interesting to speculate that the SI of loggerheads could perform respiratory functions.
We initially aimed to observe Paneth, enteroendocrine and goblet cells; however, only the latter were identified. These cells showed histochemical characteristics that will be discussed in more detail below.
The submucosa evaginates toward the lumen forming plicae (Figs. 1A and 1E). The presence of plicae in the digestive apparatus is one relevant characteristic of the loggerhead SI. In the case of marine turtles, the physiological reasons for the presence of plicae are not confirmed; however, in the duodenum, one can macroscopically observe structures or elevations of the mucosa that are similar to a honeycomb, and these structures or elevations may not only contribute to the compaction of food but also increase the contact surface, possibly due to the lack of intestinal villi in turtles (Wyneken, 2001).
The multiple ducts with the appearance of lymphatic vessels are a feature of the SI of loggerhead hatchlings. One possibility of the presence of these ducts may be explained by the transport of chylomicrons through the lymphatic vessels (Tso and Balint, 1985). Chylomicrons are known as cholesterol transporters (Tso and Balint, 1986), and loggerheads are high consumers of cholesterol (Kakizoe, 2004) possibly due to a diet rich in crustaceans (Ernst and Lovich, 2009).
The groups of pale cells observed on the submucosa and between the circular and longitudinal muscle layers (Figs. 1B and 1F) may correspond to the submucosal and myenteric plexuses, which act as important modulators in peristalsis and the compaction of intestinal contents (Holmberg et al., 2002). In the freshwater turtle Pseudemys scripta (syn. Trachemys scripta elegans), the intrinsic enteric innervation was described by Timmermans et al., (1991), but no data exist on sea turtles to conduct a comparative study.
Histochemistry
The combination of MPSs has been discussed as an adaptive aspect (Park et al., 2001) that allows for lubricating and cleaning the mucosa (Moitra et al., 1989) due to the capacity to favor the interactions between water and mucosa (Singh et al., 1974). Rogers (1961) reported that 1 g of MPSs can bind to 200-500 g of water.
The gamma-metachromasia reaction, observed in the secretory granules of the GCs in the cranial portion, is related to the presence of electronegative components (polyanions), particularly sulfated esters that favor the accumulation or polymerization of the cationic components of the Giemsa and TB staining (Pearse, 1960; García del Moral, 1993). It has been suggested that AMPSs, especially sulfated AMPSs, act as a defense barrier, thus protecting the intestine from potential pathogens and from digestive enzymes (Robertson and Wright 1997). This is compatible with other studies that indicated the GCs of the intestinal region that were densely populated with microorganisms mainly secreted acid mucins (Nieuw et al. 1998). Furthermore, sulfated AMPSs are more resistant to bacterial glycosidase degradation, and they display a stronger inhibitory effect on bacterial growth than non-sulfated AMPSs (Conour et al., 2002).
Furthermore, the affinity of GC granules to MS staining is associated with the reactive aldehydes obtained after a high oxidation with chromic acid, demonstrating both glycogen and/or neutral MPSs (Pearse, 1960). Glycogen has been reported in the goblet cells of rats, and the role of glucose as a precursor in the synthesis of other carbohydrates in the Golgi complex has been discussed (Neutra and Leblond, 1966). The cytoplasmic vesicles observed with MS are suggestive of the Golgi apparatus cisternae system (Rambourg et al., 1969).
The nature of the cells with the granules that reacted with the silver impregnation stains, Grimelius and M-F, was not fully determined. Ahmed et al., (2009) performed Grimelius impregnation techniques in Varanus niloticus to demonstrate the presence of enteroendrocrine cells, a type of epithelial cell. Moreover, in mammals, the argentaffin and argyrophilic reactions have been associated with molecules capable of reducing silver nitrate, such as biogenic amines (mainly 5-hydroxytryptamine and dopamine) (Lundqvist et al., 1990), which are synthesized by mast cells (Kushnir-Sukhov et al., 2007) and enteroendrocrine cells (Grimelius, 2004). In C. caretta, due to the location of these cells in the submucosa and their morphology, these cells could possibly belong to mast cells; however, they are typically reactive to TB (Chiu and Lagunoff, 1972), which was not observed in this study. The possible presence of other gland cells such as Paneth cells is speculative, as they have not been reported in other turtles such as the tortoise Testudo graeca (Perez-Tomas et al., 1990), or even in other reptiles (Ferri et al. 1976, Kotze et al. 1992).
The fact that the animals used were hatchlings complicates the interpretation of our results. In this regard, complementary immunohistochemical studies should be performed to analyze both mast and Paneth cells.
Conclusions
The SI of loggerhead hatchlings is a peculiar organ, mainly due to the pseudostratified epithelium and multiple lymphatic vessels. The histological and histochemical differences along the SI indicate that the functions performed by both portions are different. For example, sulfated AMPSs, which form a microorganism defense barri er, are mainly in the cranial portion. It is necessary that these results be compared using different age groups.
New data and hypotheses, such as a possible respiratory function, have been presented; however, further ultrastructural studies with electron microscopy should be performed. Similarly, complementary techniques to determine the presence of Paneth and enteroendocrine cells will be necessary. As regards gland secretion, lectin histochemistry will clarify the biochemical nature of the studied carbohydrates. With respect to the immune system, immunohistochemical studies aimed at determining the presence of cells such as intraepithelial lymphocytes, dendritic cells and migrating lymphocytes would elucidate the mechanisms of mucosal immunity. Regarding chylomicron physiology, similar studies to those of Tso and Balint (1985) should be performed. Perspective studies simply require histological descriptions; thus, this study serves as an important foundation for future research.
We lastly acknowledge the limitations of this study, such as the number of individuals, the selected age groups and the type of tissue preservation. This research provides a valuable histological effort into the comprehension and application of knowledge in the conservation of marine turtles.











 texto en
texto en 


