Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Abanico veterinario
versión On-line ISSN 2448-6132versión impresa ISSN 2007-428X
Abanico vet vol.13 Tepic ene./dic. 2023 Epub 09-Jun-2023
https://doi.org/10.21929/abavet2023.4
Revission Literary
Tilapia, high socio-economic cichlid fish, as host of trematode parasites with zoonotic potential
1Universidad Autónoma del Estado de Hidalgo, Instituto de Ciencias Agropecuarias, Área Académica de Medicina Veterinaria y Zootecnia, México.
2Microbiología en Salud Humana. Facultad de Estudios Superiores Cuautitlán. Universidad Nacional Autónoma de México. Avenida 1 de mayo S/N, Campo Uno, Cuautitlán Izcalli, CP 54743, Estado de México, México.
The production and consumption of tilapia (Cichlidae) is very popular in the world, so the safety of the meat produced must be ensured given the potential risk of zoonotic transmission of parasites; For this reason, the objective of this work was to carry out a bibliographic review on the prevalence, distribution and hosts that intervene in the life cycle of trematode helminths that can be transmitted zoonotically in the consumption of tilapia. The bibliographic review was carried out with six specialized search engines. 1,044 articles were analyzed, of which 113 included epidemiological data. Tilapia was reported as the intermediate host of 15 species of trematodes that affect humans, 6 parasites were reported at the genus level and 2 parasites at the family level. The described flukes belong to the families Heterophyidae and Opisthorchiidae. Reported prevalences range from 1% in infections by Haplorchis pumilio and Centrocestus formosanus to 93.64% in multiparasitic infections by Haplorchis yokogawi, Pygidiopsis genata and Phagicola ascolonga. Although the biodiversity of documented helminths in tilapia is abundant, the available information is still insufficient, situating tilapia as a potential transmitter of these helminths in humans.
Keywords: tilapia; trematodes; zoonotic parasites; aquaculture; fisheries; host
La producción y consumo de tilapias (Cichlidae) es muy popular en el mundo, por lo que debe asegurarse la inocuidad de la carne producida ante el riesgo potencial de la transmisión zoonótica de parásitos; por ello, el objetivo de este trabajo fue realizar una revisión bibliográfica sobre la prevalencia, distribución y hospederos que intervienen en el ciclo de vida de los helmintos trematodos que pueden transmitirse de manera zoonótica en el consumo de la tilapia. La revisión bibliográfica se llevó a cabo con seis motores de búsqueda especializada. Se analizaron 1,044 artículos, de los cuales 113 incluían datos epidemiológicos. La tilapia fue reportada como el hospedero intermediario de 15 especies de trematodos que afectan al humano, 6 parásitos se reportaron a nivel de género y 2 parásitos a nivel de familia. Los trematodos descritos pertenecen a las familias Heterophyidae y Opisthorchiidae. Las prevalencias reportadas oscilan de 1% en infecciones por Haplorchis pumilio y Centrocestus formosanus hasta 93.64% en infecciones multiparasitarias de Haplorchis yokogawi, Pygidiopsis genata y Phagicola ascolonga. Aunque la biodiversidad de helmintos documentados en la tilapia es abundante, aún es insuficiente la información disponible, situando a la tilapia como un transmisor potencial de estos helmintos en el humano.
Palabras clave: tilapia; trematodos; parásitos zoonóticos; acuacultura; pesca; hospederos
INTRODUCTION
There are many papers that describe the nutritional and economic importance of worldwide fishing and aquaculture (Adugna et al., 2020; Chibwana et al., 2020; Okoye et al., 2014). This activities annually produce more than 179 million tons of fish (FAO, 2021) and one of the most produced, is the fish named “tilapia”, a term that is used to refer to cichlid fishes of the genera, Oreochromis, Sarotherodon and Tilapia. In Mexico, during the last 10 years, an increase in tilapia production has been reported, with an average annual growth of 3.1% (Huerta-Mata & Valenzuela-Oyadener, 2019; SIAP, 2022). Due to the expansion of fishing and aquaculture, requirements of food safety strategies are mandatory, v. gr., the presence of potential pathogen microorganism, as the parasites, compromises the safety of fishery products (Ananda-Raja & Jithendran, 2015; Williams et al., 2020a).
Among the helminths, the most widely studied for their zoonotic transmission from fish meat are the trematodes, colloquially named “flukes”, from the families Heterophyidae (intestinal flukes), Echinostomatidae (intestinal flukes), and Opisthorchiidae (liver flukes), which are represented by the species Clonorchis sinensis, Opisthorchis viverrini, O. felineus, Metagonimus yokogawai and Heterophyes spp. (Lima dos Santos & Howgate, 2011; Wiriya et al., 2013). The tapeworms Diphyllobothrium latum and D. pacifica as well as the nematodes Capillaria philippinensis, Gnathostoma hispidum, G. spinigerum, G. doloresi, G. nipponicum, Pseudoterranova decipiens, Contracaecum osculatum and Anisakis simplex are also studied. As example of medical and economical importance, there are annually more than 1,000 clinical cases of anisakidosis in Japan transmitted by the ingest of more than 100 species of fishes that are eaten without cooking (Bao et al., 2019). Gnathostomosis is another helminthic disease reported frequently in tourists (Bravo and Gontijo, 2018). Although more than 1,000 cases of gnathostomosis had been recorded in Mexico between 1970 and 1999 (Lamothe-Argumedo, 1999), there are not enough systematic studies of prevalence and distribution of helminths with zoonotic potential that parasitized fish of economic importance (Carrique-Mas & Bryant, 2013; Pritt, 2015).However, it is known that in in Asia, 18 million people are infected by trematodes (Mahmoud et al., 2016; Wiriya et al., 2013) and in the world there are 500 million people at risk of infection (Chi et al., 2008). In this way, considering the importance of helminths and the commercial level of tilapia (Gulelat et al., 2013; Mahmoud et al., 2016; Soler Jiménez et al., 2016; Watterson et al., 2012); the objective of this work was to carry out a literature review about the prevalence and distribution of trematode helminths that can be transmitted as zoonosis during the tilapia production chain.
MATERIAL AND METHODS
Bibliographic research
The review was carried out with search engines based on the use of the keywords: zoonotic parasite, trematode, foodborne parasites, Oreochromis and tilapia. The variants that make references to the zoonotic parasite disease were centrocestiosis (Centrocestus), clonorchiosis (Clonorchis), opisthorchiosis (Opisthorchis), heterophiosis (Heterophyes) and haplorchiosis (Haplorchis). Taking in account these words, the inclusion as a whole, was structured as shown, “(Centrocestus or centrocestiosis) and (Oreochromis or tilapia)” for each organism / parasitic disease. Six specialized bibliography search engines were used (ScienceDirect, PubMed, Primo, CONRICyT, LILAES and AJOL), a total of 1,044 search results were obtained to which exclusion criteria were applied, eliminating zoonotic parasitic diseases in other taxa of parasites and from fish that were not within the scope of this review. Replicas were eliminated and studies were defined as those that included basic epidemiological data, prevalence and distribution. The purification delimited 80 bibliographic sources for the qualitative analysis and 33 bibliographic sources referring to prevalence that was part of the information analyzed in this review, thus, a total of 113 papers were analyzed. Subsequently, the information was captured in tables to data presentation in geographic distribution map, using the free access computer program RSstudio (Boston, MA, USES).
RESULTS AND DISCUSSION
General life cycle of Trematodes
Flukes are helminths taxonomically located within the group of flatworms. In general, flatworms meet three characteristics: they are acellomate, protostomate and triblastic (Negrete & Damborenea, 2017). Trematodes include a number of parasitic species of animals that accidentally reach humans. Most trematodes have complex life cycles involving multiple hosts (Fang et al., 2018). The general life cycle of trematodes begins with the monoecious adult worm that is established in the viscera of the digestive system of a vertebrate. The adult worms lay eggs that use the host's faeces as a vehicle for dispersal. The eggs are dispersed in the water and from there, the first larva emerges, which is called miracidium and it is mobile. The miracidium stage looks for the first intermediate host (gastropod mollusk), in host develops into a sporocyst and this, in turn, into redias that reproduce asexually, finally mollusk expels cercariae stage. Cercariae swim and disperse in the aquatic environment until they find the second intermediate host, which can be a fish (Lima dos Santos & Howgate, 2011; Zhao-Rong et al., 2005). In the skeletal muscle of the second intermediate host, the cercariae become metacercariae, which are surrounded by a chitin wall that allows them to resist climatic changes or simply the passage through the digestive tract of the definitive host that in a natural way it is a vertebrate animal with ichthyophagous habits (Mutengu et al., 2018). In the definitive host, the metacercariae are released from the capsule and establish themselves in some of the viscera of the digestive system, to become adult worm stage (Galaktionov & Dobrovolskij 2003; Burton et al., 2019). Foodborne zoonosis are infections that affect humans and are acquired through the ingestion of food of vertebrate animal origin (Carrique-Mas & Bryant, 2013). This type of food can transmit the infection to humans because it comes from animals that are part of the life cycle of parasites; for example, from raw fish meat. In particular, fish flukes have life cycles with multiple hosts (Fang et al., 2018), including a definitive single host for the adult worm stage and one or more intermediate hosts, which harbor the different stages of parasite development (Chibwana et al., 2020; Hung et al., 2015). Gastropod mollusks are primary intermediate hosts (Chi et al., 2008), while fish are secondary intermediate hosts and have a transcendental role in the transmission of the parasite through the trophic chain (Koinari et al., 2013).
Cercariaes in tilapia environments
Given the importance of gastropods mollusks in the life cycle of trematodes, removing snails from ponds for tilapia production is recommended (Kang et al., 2013). In the aquaculture and fishing of has been identified some species of gastropods as primary host of trematodes. Table 1 shows the prevalence of cercariae found in several mollusk involved in the tilapia chain production. The main data is reported from Asia. For example, in Vietnam, where ponds and rice plots converge, at least 15 or more species of gastropods have been described, such as Melanoides tuberculata, Bithynia fuchsiana and Stenothyra messageri with a cercarial infection prevalence of 8.9%, 6.4% and 1.5%, respectively (Madsen et al., 2015). The trematodes Haplorchis pumilio has been isolated in fish ponds with presence of five gastropods species, one of which belongs to the Viviparidae family (Angulyagra polyzonata) and the other four to the Thiaridae family: Melanoides tuberculata, Thiara scabra, Tarebia granifera and Sermyla requetii (Van Phan et al., 2010). Cercariae of the genus Procerovum have been recorded in the gastropods Melanoides tuberculata (0.92%) and Bithynia fuchsiana (0.11%). Opisthorchis viverrini can be found in the gastropods Bithynia siamensis goniomphalos (0.86%) and B. funiculata (0.14%) (Dao et al., 2017; Hung et al., 2015). Cercariae of Clonorchis sinensis have been recorded in the families Hydrobiidae, Bithyniidae, Melaniidae, Assimineidae and Thiaridae (Zhao-Rong et al., 2005), particularly in the species Alocinma longicornis (27%), Bithynia fuchsianus (8%) and Parafossarulus striatulus (8%) (Petney et al., 2013; Zhao-Rong et al., 2005). The cercariae of Heterophyidae and Echinostomatidae families are established in the mollusks Pomacea canaliculata, Bellamya aeruginosa and Cipangopaludina Oncomelania (Kang et al., 2013). Heterophyes heterophyes cercariae is related to four families of gastropods as primary hosts (Potamididae, Melaniidae, Pleuroceridae and Littorinidae), although the most recurrent specie is Pirenella conica (Chai, 2014; Chai & Jung, 2017). Similarly, loads of Clinostomun complanatum have been reported in Radix swinhoei as the primary host, with a prevalence of 0.62 (Wang et al., 2017a). In America, reports of cercariae were found from Costa Rica, where the gastropods Melanoides turricula, Pomacea flagellata, Haitia cubensis and the bivalve Anodontiles luteola have been recorded as intermediate hosts for Centrocestus formosanus (Cortés et al., 2010).
Table 1 Prevalence of cercariae associated with the production chain and capture of tilapia for consumption
| Fluke | Primary intermediary host | Continent distribution | Prevalence of cercariae | Secondary intermediary host | Reference |
| Opisthorchis viverrini | Bithynia siamensis goniomphalos Bithynia funiculata | Asia (Vietnam) | 0.14 0.86 | Oreochromis niloticus | (Dao et al., 2017) |
| Clonorchis sinensis | Parafossarulus striatulus Alocinma Longicornis Bithynia fuchsianus | Asia (China) | 3-8 27 8 | Tilapia mossambica Oreochromis Mossambicus Oreochromis mossambicus | (Zhao-Rong et al., 2005) (Petney et al., 2013) |
| Haplorchis sp., Procerovum varium e Indefinidos | Bithynia fuchsiana y Melanoides tuberculata | Asia (Vietnam) | 0.11-0.92 | Oreochromis niloticus | (Hung et al., 2015) |
| Haplorchis pumilio | Melanoides tuberculata, Bithynia fuchsiana y Stenothyra messageri | Asia (Vietnam) | 1.5-8.9 | Oreochromis niloticus | (Madsen et al., 2015) |
| Clinostomum complanatum | Radix swinhoei | Asia (China) | 0.62 | Tilapia zillii | (Wang et al., 2017a) |
The study of gastropods mollusks are important, since this organism are extremely resistant to abiotic factors, such as Pirenella conica, which is resistant to salinity between 15 and 80 ppm (Chai, 2014; Chai & Jung, 2017; Hung et al., 2013). One of the most recurrent primary hosts is the gastropod mollusk Melanoides tuberculata (Cortés et al., 2010; Hung et al., 2013; Petney et al., 2013; Pinto et al., 2014; Zhao-Rong et al., 2005), which is resistant to desiccation, low oxygen levels, and extreme salinity, also, resist a temperature lower than 18 °C (Fleming et al., 2011). In culture ponds, the vegetation surrounding and the oligotrophic environments promote the establishment of different populations of gastropods (Chi et al., 2008; Cortés et al., 2010). Due to these characteristics, the sanitary management of gastropods mollusks requires special attention.
Metacercaria stage isolated in fishing tilapia
Tilapia has been reported as a transmitter of zoonotic flukes, becoming a public health problem and, in some cases, spoils the commercial perception of aquaculture (Adugna et al., 2020; Chibwana et al., 2020). Table 2 shows the prevalence of metacercaria in different tilapia species; the table also shows that cercariae have no predilection for the anatomical region of the fish. The analyzed reports include different trematode species, such as, Heterophyes heterophyes metacercariae which is parasite of Tilapia simonis, T. nilotica and T. zillii (Chai, 2014; Chai & Jung, 2017). In natural water bodies, metacercariae of Opisthorchis felineus, O. viverrini and Clonorchis sinensis have been collected from Oreochromis and Tilapia (Petney et al., 2013; Wang et al., 2018; Williams et al., 2020a; Zhao-Rong et al., 2005), while metacercariae of Centrocestus formosanus have been recovered from Oreochromis niloticus produced in a recreational artificial lake in Belo Horizonte, Brazil (Pinto et al., 2014). Prevalence of Opisthorchis viverrini metacercariae in Oreochromis niloticus young tilapia inhabiting of a lake in Binh Dinh province, Vietnam, was of 18.8% (Dao et al., 2017), while in a dam from Zimbabwe, the prevalence of Clinostomum metacercariae was of 62.8% in Oreochromis mossambicus (Mutengu et al., 2018). In another context, in the Lake Agulu, Nigeria, the prevalence of Clinostomum tilapiae metacercariae in Tilapia zillii was 1.54% (Okoye et al., 2014).
Metacercariae and concomitant parasite infections in tilapia fish
Co-infections in tilapia by different taxonomic groups is a common event. The study of concomitant infections is pertinent because the risk of morbidity and mortality in the production of fish for commercial and consumption purposes. Concomitant infection between two etiological agents from different taxonomic groups or multiple etiological agents from the same taxonomic group have been documented. In the first case, we can mention as an example, the infection between the ciliated protozoa Trichodina heterodentata or Ichthyophthirius multifiliis with the bacterium Streptococcus iniae (Abdel- Latif et al., 2020). Regard to helminths, the co-infection between the nematode Contracaecum multipapillatum (51.8% prevalence) and the trematode Heterophyes sp. (19.6% prevalence) in Tilapia zillii or Oreochromis leucostictus was documented in Lake Neivasha in Kenya (Otachi et al., 2014). In addition, concomitant infection between larvae of Contracaecum sp. nematode (5.48%) and metacecariae of Clinostomum sp. (27.39%) were found in the mesentery, pericardial area and branchial cavity of Oreochromis niloticus resident of the Koka water reserve in Ethiopia (Gulelat et al., 2013). In the case of multiparasitism, the findings reported in Tilapia nilotica and Tilapia zillii from the Lake Manzala (brackish water) and from the Nile River (fresh water) can be cited as example. In the first case, the frequency of infection was 64.9%, while in the second case it was 17.6% and the metacercariae collected were from the genera Heterophyes heterophyes, H. aequalis, Pygidiopsis genata, Haplorchis yokogawai, H. pumilio, Phagicola ascolonga and Stictodora tridactyla (Elsheikha & Elshazly, 2008b; Hegazi & Abo-elkheir, 2014). These data suggest that tilapia is an organism that tolerates multiparasitism. Also, some parasites such as Clinostomum sp. showed a higher prevalence in concomitant infection than when recovered individually, this may be because some microorganisms arise as opportunistic infections in organisms that have primary infections (Fajer-Ávila et al., 2017).
Table 2 Reports of metacercariae in tilapia. Geographic and anatomical distribution of parasites, only the reports that have prevalence are included
| Tilapia | Fluke | Distribution | Prevalence of metacercariae | Anatomical distribution | Reference |
| Oreochromis niloticus | Centrocestus formosanus Haplorchis pumilo | Vietnam, China | 11.8%-12.5% | ca, mu, pi, al, br, es | (Chi et al., 2008) |
| Oreochromis niloticus | Opisthorchis viverrini | Thailand, Cambodia, Laos, Vietnam | 18.8% | - | (Dao et al., 2017) |
| Oreochromis niloticus, T. zillii | Heterophyes heterophyes, H. aequalis, Pygidiopsis genata, Phagicola sp., Haplorchis sp., Stictodora sp. | Egypt | 16.4%-17.6% | mu | (Elsheikha & Elshazly, 2008a) |
| Oreochromis niloticus | Heterophyidae Echinostomatidae | China | 1.5% | ca, br, mu, al, pi, es | (Kang et al., 2013) |
| Oreochromis niloticus, T. zillii | Heterophyes heterophyes, H. aequalis, | Egyp | 30% -33.3% | mu, ca | (Lobna et al., 2010) |
| Pygidiopsis genata, Ascocotyle (Phagicola) ascolonga, Haplorchis yokogawi | |||||
| Oreochromis niloticus | Centrocestus sp. | Egypt | 10% | br | (Mahmoud et al., 2016) |
| Oreochromis niloticus | Centrocestus formosanus | Brazil, Egypt, Vietnam, Saudi Arabia | 31.1% | br | (Pinto et al., 2014) |
| Oreochromis niloticus | Clonorchis sinensis, Haplorchis pumilio, H. taichui, Centrocestus formasanus | Vietnam, Korea, China, Thailand | 2%-10% | - | (Van De et al., 2012) |
| Oreochromis niloticus, Oreochromis niloticus | Stellantchasmus falcatus, Haplorchis pumilio, Procerovum varium | Thailand, Lao, Cambodia, Vietnam | %-50% | al | (Wiriya et al., 2013) |
| Oreochromis niloticus | Centrocestus formosanus | Costa Rica | 1026(total number recovered) | br, al y te | (Cortés et al., 2010) |
| Oreochromis niloticus T. zillii | Heterophyes sp., Pygidiopsis genata, Haplorchis pumilio, Phagicola sp., Stictodora tridactyla | Egypt, Palestine, Hawaii, Ukraine, Canada, Alaska | 42.6% 64.9% | - | (Hegazi & Abo- elkheir, 2014) |
| Oreochromis niloticus, O. mossambicus | Haplorchis taichui | Laos, Thailand, Cambodia, Vietnam | 0% | - | (Kopolrat & Sithithaworn, 2015) |
| Tilapia sp. | Clonorchis sinensis | China | 0% | mu | (Wang et al., 2017b) |
| Oreochromis niloticus | Heterophyes sp. | Kenya | 6%-8% | - | (Ojwala et al., 2018) |
| Oreochromis sp. | Haplorchis pumilio | Vietnam, China | 3%-15.6% | mu, hu, pi, ca, cau | (Chi et al., 2009) |
| Oreochromis aureus | Centrocestus formosanus | United States | 0% | - | (Fleming et al., 2011) |
| Oreochromis niloticus | Haplorchis sp., Procerovum varium | Vietnam | 2.19%-23% | - | (Hung et al., 2015) |
| Oreochromis niloticus | Haplorchis pumilio | Vietnam | 32% | - | (Madsen et al., 2015) |
| Oreochromis mossambicus | Clinostomum sp. | Zimbabwe | 62.80% | cb, pi, oj | (Mutengu et al., 2018) |
| Oreochromis leucostictus Tilapia zillii | Heterophyes sp. | Kenya | 19.6-51.8% | cp, br | (Otachi et al., 2014) |
| Oreochromis niloticus | Haplochis taichui | Vietnam | 24% | - | (Van Phan et al., 2010) |
| Oreochromis niloticus | Clinostomum sp. | Ethiopia | 32.4%-58.8% | cbr, cp | (Adugna et al., 2020) |
| Tilapia guinensis | Clinostomum complanatum | Nigeria, Korea, Japan | 39.99% | cb, cbr, hu, oj, mu, cp, cab, mes, vis, vn | (Echi et al., 2009b) |
| Sarotherodon melanotheron | Clinostomum complanatum | Nigeria, Korea, Japan, Ghana | 20.80% | cb, pi, oj | (Echi et al., 2009a) |
| Oreochromis niloticus | Clinostomum sp. | Ethiopia | 5.48-27.39% | mes, cp, cbr | (Gulelat et al., 2013) |
| Oreochromis niloticus | Clinostomum sp. | Benin | 6.17% | Pi, br, in, cb | (Sèdogbo et al., 2019) |
| Tilapia zillii | Clinostomum sp. | Nigeria | 1.54% | cab | (Okoye et al., 2014) |
| Oreochromis niloticus | Clinostomum sp. | Uganda | 22% | pi, es | (Walakira et al., 2014) |
Abbreviations: ca (head), mu (muscle), pi (skin), al (fins), br (gills), es (scales), est (stomach), in (intestines), te (integument), hi (liver), ri (kidney), hu (bone), cau (caudal region), cb (oral cavity), oj (eyes), cbr (branchial cavity) cp (pericardial cavity), cab (abdominal cavity), mes (mesentery), vis (viscera) and vn (swim bladder)
Metacercariae in tilapia fish farms
Prevalence studies in fish farms are of particular interest to determine the prevalence and distribution of infectious agents that could represent a risk to cause disease in humans or domestic animals. Most of the articles analyzed showed data on Oreochromis tilapia from Asian countries. In Vietnam, a 32% prevalence of metacercariae of Haplorchis pumilio was found in adult and hatchlings (Chi et al., 2008; Madsen et al., 2015) and a 24% of prevalence was found for H. taichui during the months of December and January (Van Phan et al., 2010). Metacercariae of the genus Clinostomun were found in the skin, gills, intestine and oral cavity of tilapia from Africa, particularly in the Republic of Benin (6.17%), Uganda (22%) and Ethiopia (32.4%), where Clinostomiun was found concomitantly (58.8%) with the nematode Contracaecum sp. larvae (Adugna et al., 2020; Cortés et al., 2010; Sèdogbo et al., 2019; Walakira et al., 2014), The presence of metacercariae of Centrocestus formosanus has been documented in young fish (fingerlings) with a counting of 1,026 larvae in Costa Rica (Cortés et al., 2010) and in Vietnam with a prevalence of 11.8%, where C. formosanus also occurred in co-infection with Haplorchis pumilio (Chi et al., 2008). Other metacercariae, from the Heterophyidae and Echinostomatidae families, have been found with a prevalence of 1.5% in mono- and polyculture ponds in Guangdong, China (Kang et al., 2013).
Comparative studies between fishing and aquaculture systems
There are numerous studies in the field of parasitology to try to define the behavior of parasitosis in free-living populations and crowded populations. However, it is striking that only was found some studies carried out in Vietnam and Thailand that address this comparison. In these works, Oreochromis niloticus tilapia were in captivity in farms or aquaculture ponds and the obtained data were compared with those obtained from free- living fish. In three studies (Vietnam), fish were found to have single infections with Haplorchis pumilio or concomitant infections with Procerovum varium or Centrocestus formosanus. The prevalence of infection in free-living animals was 14.3%, while in farms it was 52.8%, considering that the prevalence found in the farm was always higher (Hung et al., 2015). In contrast, in a study carried out in Thailand, three species of metacercariae (Stellantchasmus falcatus, Haplorchis pumilio, and Procerovum varium) were recorded in free-living tilapia, while no trematodes were found in fish collected from cages and ponds (Wiriya et al., 2013). The high prevalence of aquaculture populations may be due to the high stocking density that is managed in some systems.
Definitive hosts of trematodes transmitted by tilapia
The presence of definitive hosts that live around the bodies of water are indicators that the life cycle of trematodes can be completed and perpetuated in the environment (Horak et al., 2019). In the life cycle of trematodes, where tilapias intervene as secondary intermediate host, numerous species of piscivorous birds have been identified as definitive host. For example, the African aninga bird (Anhinga rufa) is the definitive host of Clinostomum (Mutengu et al., 2018). Another type of definitive hosts are the so-called accidental. Prevalence reports of trematodes associated to tilapia in accidental hosts is unusual; in most cases, the finding of adult worms in unusual hosts is fortuitous.
Table 3 Prevalence of zoonotic trematodes in accidental hosts that are associated with the production and capture of tilapia
| Trematode | Secondary intermediary host | Host | Adult parasite prevalence | Distribution | Reference |
| Heterophyes heterophyes | Tilapia nilótica, Tilapia zillii | Jackal, Fox, Dog | 14.2%, 33.3%, 2.5% | Korea | (Chai, 2014) |
| Clonorchis sinensis | Oreochromis sp. Tilapia | Shrimp | 3% | Australia | (Wang et al., 2018) |
| Heterophyes heterophyes, H. aequalis, Pygidiopsis genata, Haplorchis yokogawai Phagicola ascolonga | Tilapia nilotica, T. zillii | Dog | 19.4%, 15.4%, 18%, 12% and 11.4% (Respectively for the reported parasites) | Egypt | (Elsheikha & Elshazly, 2008b) |
| Haplorchis sp., Procerovum varium | Oreochromis niloticus | Dog, cat, pig | 32.7%, 49%, 13% | Vietnam | (Hung et al., 2015) |
| Haplochis taichui | Oreochromis niloticus | Cat, dog | 70.2% y 56.9% | Vietnam | (Van Phan et al., 2010) |
Table 3 shows the prevalence reports of zoonotic trematodes in accidental hosts that are associated with the production and capture of tilapia. Vertebrate wildlife animals, as well as domestic animals that occasionally include tilapia in their diet, can accidentally become a definitive host by consuming metacercariae that may be found in tilapia (Chai, 2014; Elsheikha & Elshazly, 2008b; Wang et al., 2017b). In general, it has been observed that the pig (Sus scrofa) can be definitive host of Haplorchis taichui and Procerovum varium (Hung et al., 2015; Van Phan et al., 2010). One of the most studied domestic animals is the dog (Canis familiaris), perhaps due to its social closeness to humans. Dog puppies can act as hosts for Heterophyes heterophyes, H. aequalis, Pygidiopsis genata, Haplorchis sp., Phagicola sp., Stictodora sp., Ascocotyle (Phagicola) ascolonga, and Haplorchis yokogawai (Elsheikha & Elshazly, 2008a; Hung et al., 2015; Lobna et al., 2010; Van Phan et al., 2010). The identification of the final hosts in the transmission of zoonotic parasites is a useful tool for the comprehensive control of parasite loads.
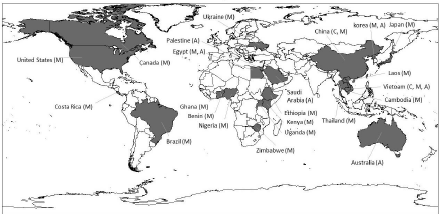
Geographic distribution of trematodes transmitted by tilapia
The worldwide distribution of trematodes transmitted by the consumption of tilapia meat is shown in figure 1. Undoubtedly, several studies have been carried out in the Asian continent to determine the prevalence and distribution of trematodes with zoonotic potential, perhaps motivated because the consumption of raw fish meat is common. In Vietnam, China, Thailand, Laos, Philippines and India, the genera Centrocestus, Clonorchis, Echinostoma, Haplorchis, Heterophyes, Opisthorchis, Phagicola, Procerovum, Pyigidiopsis, Stellantchasmus and Stictodora have been described (Chi et al., 2008; Dao et al., 2017; Hegazi & Abo-elkheir, 2014; Hung et al., 2015; Kang et al., 2013; Van De et al., 2012; Wang et al., 2017b; Wiriya et al., 2013). In the African continent, usually characterized by its abundant biodiversity, the trematodes Ascocotyle (Phagicola) ascolonga, Haplorchis pumilio, H. yokogawai, Heterophyes aequalis, H. heterophyes, Pygidiopsis genata and Stictodora tridactyla have been recorded in Egypt (Elsheikha & Elshazly, 2008b, 2008a; Hegazi & Abo-elkheir, 2014; Lobna et al., 2010); while, in Nigeria, Kenya, Zimbabwe, Ethiopia, Benin and Uganda, it has been documented the presence of the genera Clinostomum (Adugna et al., 2020; Echi et al., 2009a; Mutengu et al., 2018; Okoye et al., 2014; Sèdogbo et al., 2019; Walakira et al., 2014), Centrocestus (Mahmoud et al., 2016) and Heterophyes (Ojwala et al., 2018; Otachi et al., 2015). In the European continent, Clonorchis sinensis, Heterophyes dispar and H. heterophyes are distributed in Greece, Italy, Turkey, France, Spain, Russia and Ukraine (Chai, 2014; Chai & Jung, 2017; Hegazi & Abo-elkheir, 2014; Hung et al., 2013; Wang et al., 2018). In the American continent, the epidemiological studies of trematodes on fish are still insufficient and, the occurrence of zoonotic parasites is documented in gastronomic food of Asian origin (Castellanos-Garzón et al., 2019; Leroy et al., 2017); so, the need of conducting safety studies of fish meat used as food source is important (Gutiérrez-Jiménez et al., 2019). There are reports of Centrocestus formosanus in Brazil, Costa Rica and the United States (Cortés et al., 2010; Fleming et al., 2011; Pinto et al., 2018). In Mexico, the studies of parasites recovered from fish are focus at the southwestern region of the country. In the states of Veracruz, Oaxaca and Puebla, 39 families of helminths have been described that can infect 35 species of fish. Centrocestus formosanus can develop in 16 species of fish, including the cichlids Astatherops robertsoni, Cichlasoma fenestratuma, Cichlasoma urophthalmus and Vieja synspila. In Chiapas, 72 species of helminths have been identified in 54 species of freshwater fish, 10 of which are cichlids, and among them the trematode Clinostomum complanatum was identified in fish of the genus Vieja (Salgado-Maldonado et al., 2005, 2011).
Influence of socioeconomic activities on trematode distribution
The distribution of zoonotic parasites that infect tilapia (figure 1), is dependent on multiple factors that can influence their dispersion. This is relevant because fishing and aquaculture of tilapia take place in more than 100 countries around the world. The main tilapia producing countries are China with 1.8 million tons per year, Indonesia with 1.1 million tons and Egypt with 875 million tons (Abdel-Latif et al., 2020). However, the prevalence and biodiversity of trematode flukes are associated with the geographic areas where intermediate and definitive hosts converge in the same trophic chain. Socio- economic activities, such as polyculture, wet markets, the unregulated sale of fish hatchlings (Tesana et al., 2014), the consumption of raw food, tourism and migratory flow (Chai & Jung, 2017), among others, promote the occurrence and dispersal of zoonotic parasites (Carrique-Mas & Bryant, 2013). An example of the influence of humans on parasitic dispersal is associated with the import/export of animals for zootechnical purposes.
Trematode pathology in aquaculture of tilapia
Parasites, as their definition indicates, develop pathological problems in the host and, fish are no exception to the rule. The course of pathologies caused by parasites can culminate in economic losses for the producer (Gulelat et al., 2013) and, the severe clinic cases put at risk the food security of some areas with high fish consumption. Fish that present parasitosis are more likely to present secondary pathologies due to viruses, bacteria, or fungi (Mutengu et al., 2018). Tilapias are not exempt from presenting a clinical signs in the presence of larvae of trematodes (Echi et al., 2009b), which can be the cause of deficit in the growth of fish, culminating in some morbidity and even mortality (Adugna et al., 2020; Fang et al., 2018). For example, the Centrocestus formosanus metacercariae cause swimming alterations (curved, erratic or spiral) of tilapia (Cortés et al., 2010), in addition to edema, hemorrhage, loss of respiratory epithelium, fusion of primary lamellae, destruction of lamellae secondary disorders and distortion due to hyperplasia of the branchial cartilage (Fleming et al., 2011; Mahmoud et al., 2016), causing a decrease in respiratory capacity and death. The infection of Clinostomum complanatum metacercariae in Tilapia guinensis, trigger hemorrhages and skin damage due to penetration of the cercariae, different degrees of damage to the eyes, from exophthalmia, necrotic cells and ulceration of the coating membranes to blindness (Echi et al., 2009b).
Diagnosis of metacercariae in fish
The lifetime that a metacercaria can be in hypobiosis at muscle of fishes is variable. Haplorchis pumilio metacercariae are viable for nine weeks in Sarotherodon spilurus, but Clinostomun complanatum metacercariae can be present throughout the life of Tilapia guinensis, which is 2 to 3 years (Boerlage et al., 2013; Echi et al., 2009b). Due to this variability, the diagnosis in fish’s hosts is a difficult task. Also, diagnostic tools in aquatic organisms are frequently applied in fish farms than in wild life fish. The tools can be classified in macroscopic, microscopic, histological, microbiological, immunological and molecular, which allow follow-up from the presumptive diagnosis to the isolation and identification of the etiological agent (Sitjà-Bobadilla & Oidtmann, 2017). Regarding parasitological diagnosis, gill biopsies, skin cytology, fecal examination, and post-mortem studies are common within 6 to 8 hours after death. For endo-parasite recovery, liver, spleen and digestive tract are examined, among others (Mjakakhamis & Sagweorina, 2017). The recovery and preservation of parasites require their deposition in 70% ethanol solution for taxonomic identification, generally through morphological characteristics (Elsheikha & Elshazly, 2008b, 2008a; Sepulveda & Kinsella, 2013). The recovery of metacercariae includes the observation of meat between two glass plates against the light, using a light source of 100 watts. The isolation is achieved from an artificial digestion with pepsin, the resulting material is filtered and washed with 0.85% physiological saline solution (Diaz Camacho et al., 2002). On the other hand, it is well known that the use of molecular diagnosis is essential for the confirmation of cases and identification of the etiological agents. However, there are still insufficient diagnostic studies in tilapia that have used molecular methodology, such as the polymerase chain reaction in the identification of zoonotic trematodes. For example, in Thailand, three trematodes, Stellantchasmus falcatus, Haplorchis pumilio and Procerovum varium, were identified in Nile tilapia from the amplification of the 28S rDNA gene and the use of primers LSU-5, 1500R and 900F (Wiriya et al., 2013).
Pathology in humans
Anthropocentric changes, such as the urbanization of wilderness areas, the intensification of food production and the modernization of the market increase the risk of human exposure to unusual pathogens (Carrique-Mas & Bryant, 2013; Chi et al., 2009; Pinheiro et al., 2019; Wang et al., 2017b). Even the change or the neglect of human eating habits can be a risk in the transmission of zoonotic diseases. Consuming raw or undercooked tilapia meat can be the source of infection of helminth larvae (Wang et al., 2017b), when the human being consumes metacercariae, these larvae will develop into adult worms and, ultimately, the adults will be the origin of the pathology in humans. The manifestations of the disease can range from subclinical to polysymptomatic with different degrees of severity, depending on some factors such as parasite load, immune status, and previous exposures to the etiological agent (Chai, 2014). In general, the infection by adult worms of Clonorchis sinensis, Opisthorchis verrini and O. felineus are associated with peripheral eosinophilia with intermittent jaundice and leukocytosis in 40% of clinical cases (Zhao- Rong et al., 2005). In early infection, suppurative cholangitis extended to the parenchyma of the liver tissue is observed, causing hepatitis with the formation of micro- and macro- abscesses, whereas in chronic infection there may be cholangiocarcinoma. O. viverrini adults are associated with adenomatous hyperplasia of the biliary epithelium and thickening of the walls with fibrous connective tissue, hypertrophy and dilation of the bile ducts (Hung et al., 2013). Clonorchis sinensis parasitosis is characterized by hyperplasia and metaplasia of the intrahepatic bile epithelium, followed by periductal fibrosis (Dao et al., 2017; Hung et al., 2015; Wang et al., 2018).Clinostomum adults damage the pharynx, in the arytenoid region, oropharyngeal wall and lateral lymphatic band, causing discomfort in the throat, pain when eating food, bloody phlegm and fever (Acosta et al., 2016), becoming diagnosed as pharyngitis, laryngitis or the clinical syndrome called halzoun (Echi et al., 2009a; Fang et al., 2018; Williams et al., 2020b). Infections with adult worms from trematodes of Echinostomatidae family manifest different degrees of focal necrosis and inflammation of the intestinal mucosa, severe infections can cause eosinophilia, abdominal pain, severe diarrhea, anemia, and anorexia (Petney & Taraschewski, 2011). Infection with Heterophyes heterophyes, like other flukes of the Heterophyidae family, for example, Metagonimus, which, transmitted by freshwater carp, cause diarrhea and abdominal pain, lethargy, anorexia and weight loss, in addition, there may be erratic or extraintestinal parasitism in the heart, brain, and spinal cord (Chai, 2014; Wiriya et al., 2013). The eggs of heterophyid flukes can be carried through the bloodstream to unusual ectopic sites, producing eosinophilic granuloma in the heart, brain, and spine (Elsheikha & Elshazly, 2008b, 2008a; Hegazi & Abo-elkheir, 2014; Kang et al., 2013). An epidemiological study in Egypt documented that the risk factors for infection with Heterophyes aequalis, H. heterophyes, Pygidiopsis genata, Ascocotyle (Phagicola) ascolonga or Haplorchis yokogawaik were associated with (1) female sex (odss-ratio (OR)= 1.59), (2) be fisher (OR = 1.39) or homemaker (OR = 1.24) or (3) being in the age groups of 15 to 45 years (OR = 2.22) or 5 to 14 (OR = 1.29) (Lobna et al., 2010). In Vietnam it was found that the frequency of infection is higher in people over 19 years of age (4.2 to 53.8%) compared to those under 19 years of age (1.4 to 13.4%), likewise, the occurrence by gender was 31.1% in men, while in women it was 13% (Hung et al., 2015). In Vietnam, during the evaluation of the prevalence of Haplorchis taichui in ponds where the tilapia Oreochromis niloticus is produced, a negative effect of the ignorance of the inhabitants on the sanitary quality of the water with which the fish produce was documented, the occurrence of the parasites was 76% in ponds fed with channel water and 82% in ponds where the feed water had contact with drainage discharges (Van Phan et al., 2010).
Intervention strategies
One of the recurring activities in aquaculture to avoid parasitic infections is the elimination of intermediate hosts. The use of anthelmintic drugs in aquaculture is common; however, the development of drugs for the exclusive use of aquaculture is still insufficient. The use of 70% niclosamide directly in the water body is one strategy to parasite control by elimination of gastropod mollusks, which is the intermediary host of Opisthorchis viverrini (Tesana et al., 2014). Regarding the treatment against helminths in aquaculture, only a few drugs such as praziquantel have been used in Oreochromis niloticus (Bader et al., 2019). Praziquantel has also been used at a dose of 2.5 mg/L as an antiparasitic in target pufferfish (Sphoeroides annulatus), where a combination of praziquantel, ivermectin, pyrantel and fenbendazo was also used (Morales-Serna et al., 2018). Praziquantel has also been used at doses of 2 to 40 mg/L dispensed in feed for catfish, carp, and trout (Bader et al., 2019). Albendanzaol has also been used as an anthelmintic treatment in tilapia, however more studies are needed regarding the use of anthelmintics in aquatic organisms (Portela et al., 2020).
CONCLUSION
In this work, we described that the biodiversity of trematodes with zoonotic potential that can be transmitted by tilapia is abundant. Most of these trematodes have been described with greater occurrence in Asia, Africa and Europe, but prevalence and distribution studies are insufficient in America. It is also clear that human activities in the food industry also influence the spread of trematodes in the world, without taking into account that climatic changes and the condition of the host can also influence the prevalence of trematodes in fish. Finally, in this work we document that tilapia, a fish of high socio-economic value, without proper sanitary management can become a transmitter of trematode parasites with zoonotic potential. New studies should be carried out in tilapia with another taxonomic group of helminths, such as nematodes and cestodes to have a complete picture of the biodiversity of helminths and the risk of being transmitted to humans.
LITERATURA CITADA
ABDEL-LATIF HM, Dawood MA, Menanteau-Ledouble S, El-Matbouli M. 2020. The nature and consequences of co-infections in tilapia: A review. Journal of Fish Diseases. 43(6): 651-664. https://doi.org/10.1111/jfd.13164 [ Links ]
ACOSTA AA, Caffara M, Fioravanti ML, Utsunomia R, Zago AC, Franceschini L, Da Silva RJ. 2016. Morphological and molecular characterization of Clinostomum detruncatum (Trematoda: Clinostomidae) metacercariae infecting Synbranchus marmoratus. Journal of Parasitology. 102(1): 151-156. https://doi.org/10.1645/15-773 [ Links ]
ADUGNA M, Fishery N, Life A. 2020. The prevalence of fish parasites of Nile tilapia (Oreochromis niloticus) in selected fish farms, Amhara Regional State. Ethiop. J. Agric. Sci.. 30(3):119-128. ISSN: 2415-2382. https://www.ajol.info/index.php/ejas/article/view/198460 [ Links ]
ANANDA-RAJA R, Jithendran K. 2015. Aquaculture disease diagnosis and health management. Advances in Marine and Brackishwater Aquaculture. 1(1): 1-262. https://doi.org/10.1007/978-81-322-2271-2 [ Links ]
BADER C, Starling DE, Jones DE, Brewer MT. 2019. Use of praziquantel to control platyhelminth parasites of fish. Journal of Veterinary Pharmacology and Therapeutics. 42(2):139-153. https://doi.org/10.1111/jvp.12735 [ Links ]
BAO M, Pierce GJ, Strachan NJC, Pascual S, González-Muñoz M, Levsen A. 2019. Human health, legislative and socioeconomic issues caused by the fish-borne zoonotic parasite Anisakis: Challenges in risk assessment. Trends in Food Science and Technology. 86(11): 298-310. https://doi.org/10.1016/j.tifs.2019.02.013 [ Links ]
BRAVO F, Gontijo B. 2018. Gnathostomiasis: an emerging infectious disease relevant to all dermatologists. Anais Brasileiros de Dermatologia. 93(2):172-180. https://doi.org/10.1590/abd1806-4841.20187498 [ Links ]
BOERLAGE AS, Graat EAM, Verreth JA, De Jong MCM. 2013. Survival of heterophyid metacercaria in common carp (Cyprinus carpio). Parasitology Research. 112(7): 2759- 2762. https://doi.org/10.1007/s00436-013-3408-1 [ Links ]
BURTON B, Clint C, Thomas O. 2019. General Characteristics of the Trematoda. Chemical Zoology. 5:3-34. ISBN: 9780128137123. https://doi.org/10.1016/b978-0-12-395536-4.50008-6 [ Links ]
CARRIQUE-MAS JJ, Bryant JE. 2013. A review of foodborne bacterial and parasitic zoonoses in Vietnam. EcoHealth. 10(4):465-489. https://doi.org/10.1007/s10393-013-0884-9 [ Links ]
CASTELLANOS-GARZÓN JA, Daschner Á, Pustovrh MC, Cuellar C. 2019. Characteristics related to fish consumption and the risk of ichthyozoonosis in a Colombian population. Revista de Salud Publica. 21(6): 9-16. https://doi.org/10.15446/rsap.v21n6.69898 [ Links ]
CHAI JY. 2014. Helminth-Trematode: Heterophyes heterophyes. Encyclopedia of Food Safety. 2(1): 158-163. https://doi.org/10.1016/B978-0-12-378612-8.00156-6 [ Links ]
CHAI JY, Jung BK. 2017. Fishborne zoonotic heterophyid infections: An update. Food and Waterborne Parasitology. 8(9):33-63. https://doi.org/10.1016/j.fawpar.2017.09.001 [ Links ]
CHI TT, Dalsgaard A, Turnbull J, Tuan P, Murrell D. 2008. Prevalence of zoonotic trematodes in fish from a vietnamese fish-farming community. J. Parasitol. 94(2):423- 428. https://doi.org/10.1645/GE-1389.1 [ Links ]
CHI TT, Murrell KD, Mausen H, Khue NV, Dalsgaard A. 2009. Fishborne zoonotic trematodes in raw fish dishes served in restaurants in Nam Dinh province and Hanoi, Vietnam. Journal of Food Protection. 72(11):2394-2399. https://doi.org/10.4315/0362-028X-72.11.2394 [ Links ]
CHIBWANA FD, Mshana JG, Katandukila JV, Box PO, Box PO. 2020. A survey of fish parasites from Pangani Catchment and Lake Kitangiri in Singida, Tanzania. Tanzania Journal of Science. 46(1):42-52. ISSN: 0856-1761. https://www.ajol.info/index.php/tjs/article/view/194815 [ Links ]
CORTÉS DA, Dolz G, Zúñiga JJR, Rocha AEJ, Alán DL. 2010. Centrocestus formosanus (Opisthorchiida: Heterophyidae) como causa de muerte de alevines de tilapia gris Oreochromis niloticus (Perciforme: Cichlidae) en el Pacífico seco de Costa Rica. Rev. Biol. Trop. 58(1):1453-1465. ISSN: 0034-7744. https://revistas.ucr.ac.cr/index.php/rbt/article/view/5423 [ Links ]
DAO HTT, Dermauw V, Gabriël S, Suwannatrai A, Tesana S, Nguyen GTT, Dorny P. 2017. Opisthorchis viverrini infection in the snail and fish intermediate hosts in Central Vietnam. Acta Tropica. 170(1):120-125. https://doi.org/10.1016/j.actatropica.2017.02.028 [ Links ]
DIAZ CAMACHO SP, Willms K, Ramos M, De la Cruz Otero MDC, Nawa Y, Akahane H. 2002. Morphology of Gnathostoma spp. isolated from natural hosts in Sinaloa, Mexico. Parasitology Research. 88(7):639-645. https://doi.org/10.1007/s00436-002-0636-1 [ Links ]
ECHI PC, Eyo JE, Okafor FC. 2009a. Co-parasitism and morphometrics of three clinostomatids (Digenea: Clinostomatidae) In Sarotherodon melanotheron from a tropical freshwater lake. Animal Research International. 6(2): 982-986. https://doi.org/10.4314/ari.v6i2.48129 [ Links ]
ECHI PC, Okafor F, Eyo J. 2009b. Co-infection and morphometrics of three clinostomatids (Digenea: Clinostomatidae) in Tilapia guinensis Bleeker, 1862 from Opi lake, Nigeria. Bio- Research. 7(1):432-436. https://doi.org/10.4314/br.v7i1.45467 [ Links ]
ELSHEIKHA HM, Elshazly AM. 2008a. Host-dependent variations in the seasonal prevalence and intensity of heterophyid encysted metacercariae (Digenea: Heterophyidea) in brackish water fish in Egypt. Veterinary Parasitology. 153(1-2): 65-72. https://doi.org/10.1016/j.vetpar.2008.01.026 [ Links ]
ELSHEIKHA HM, Elshazly AM. 2008b. Preliminary observations on infection of brackish and fresh water fish by heterophyid encysted metacercariae in Egypt. Parasitol Res. 2008(103): 971-977. https://doi.org/10.1007/s00436-008-1043-z [ Links ]
FAJER EJ, Medina-Guerrero RM, Morales-Serna FN. 2017. Estrategias para la prevención y control de las enfermedades parasitarias de la tilapia. Acta Agricola y pecuaria, 3 (2): 25-31. http://aap.uaem.mx/index.php/aap/article/view/33 [ Links ]
FANG L, Xiao-Hong L, Hai-Long G, Chong-You X, Rui-Yu C, Zuo-Can H, Yao-Guang Z, Zhi-Jian W. 2018. The discovery of Clinostomum complanatum metacercariae in farmed Chinese sucker, Myxocyprinus asiaticus. Aquaculture. 495(2017):273-280. https://doi.org/10.1016/j.aquaculture.2018.05.052 [ Links ]
Organización de las Naciones Unidas para la Alimentación y la Agricultura, FAO. 2021. World Food and Agriculture - Statistical Yearbook 2021: World Food and Agriculture. Statistical Yearbook 2018. Pp. 368. ISBN 978-92-5-134332-6.https://doi.org/10.4060/cb4477en [ Links ]
FLEMING PB, Huffman DG, Bonner TH, Brandt TM. 2011. Metacercarial distribution of centrocestus formosanus among fish hosts in the Guadalupe river drainage of Texas. Journal of Aquatic Animal Health. 23(3):117-124. https://doi.org/10.1080/08997659.2011.616840 [ Links ]
GALAKTIONOV KV, Dobrovolskij AA. 2003. The Main types of trematode life cycles: The biology and evolution of trematodes. Netherlands: Springer Science+Business Media Dordrecht. Pp. 319-348. ISBN: 978-1-4020-1634-9. https://doi.org/10.1007/978-94-017-3247-5 [ Links ]
GULELAT Y, Yimer E, Asmare K, Bekele J. 2013. Study on parasitic helminths infecting three fish species from Koka reservoir, Ethiopia. SINET: Ethiopian Journal of Science. 36(2):73-80-80. ISSN: 0379-2897. https://www.academia.edu/32986068/Study_on_Parasitic_Helminths_Infecting_Three_F ish_Species_from_Koka_Reservoir_Ethiopia [ Links ]
GUTIÉRREZ-JIMÉNEZ J, Luna-Cazáres LM, Vidal JE. 2019. Malnutrition and intestinal parasites: Mexico perspectives: Handbook of Famine, Starvation, and Nutrient Deprivation. CDMX, Mexico: Springer. Pp. 1-18. ISBN: 978-3-319-40007-5 https://doi.org/10.1007/978-3-319-55387-0_7 [ Links ]
HEGAZI MA, Abo-elkheir OI. 2014. Encysted metacercariae of family Heterophyidae in infected fish in Dakahlia Governorate, an Endemic Focus in Egypt. Journal of the Egyptian Society of Parasitology. 12(2014):1-13. https://doi.org/10.12816/0007859 [ Links ]
HORAK IG, Marchiondo AA, Colwell DD, Crafford D, Kok DJ. 2019. Platyhelminthes: Parasiticide Screening in vitro and in vivo tests with relevant parasite rearing and host infection/infestation methods. London, United Kingdom: Academic Press. Vol. 2. Pp.1- 133. ISBN: 978-0-12-816577-5 https://doi.org/10.1016/B978-0-12-816577-5.00006-5 [ Links ]
HUANG S, He Y. 2019. Management of China’s capture fisheries: Review and prospect. Aquaculture and Fisheries. 4(5):173-182. https://doi.org/10.1016/j.aaf.2019.05.004 [ Links ]
HUERTA-MATA JJ, Valenzuela-Oyadener L. 2019. Perspective of the aquaculture in Chile and Mexico, productive articulation approach and business networks for international development. SSRN. 11(18):1-25. https://doi.org/http://dx.doi.org/10.2139/ssrn.3489166 [ Links ]
HUNG NM, Dung DT, Lan Anh NT, Van PT, Thanh BN, Van Ha N, Van Hien H, Canh L X. 2015. Current status of fish-borne zoonotic trematode infections in Gia Vien district, Ninh Binh province, Vietnam. Parasites and Vectors. 8(1): 1-10. https://doi.org/10.1186/s13071-015-0643-6 [ Links ]
HUNG NM, Madsen H, Fried B. 2013. Global status of fish-borne zoonotic trematodiasis in humans. Acta Parasitologica. 58(3):231-258. https://doi.org/10.2478/s11686-013-0155-5 [ Links ]
KANG L, Hedegaard Clausen J, Murrell D, Liu L, Dalsgaard A. 2013. Risks for fishborne zoonotic trematodes in Tilapia production systems in Guangdong province, China. Veterinary Parasitology. 198(1):223-229. https://doi.org/10.1016/j.vetpar.2013.08.011 [ Links ]
KOINARI M, Karl S, Ng-Hublin J, Lymbery AJ, Ryan UM. 2013. Identification of novel and zoonotic Cryptosporidium species in fish from Papua New Guinea. Veterinary Parasitology. 198(1):1-9. https://doi.org/10.1016/j.vetpar.2013.08.031 [ Links ]
KOPOLRAT, K, Sithithaworn P. 2015. Susceptibility, metacercarial burden, and mortality of juvenile silver barb, common carp, mrigal, and tilapia following exposure to Haplorchis taichui. Parasitology Research. 114(2):1433-1442. https://doi.org/10.1007/s00436-015-4326-1 [ Links ]
LAMOTHE-ARGUMEDO R. (1999). La gnatostomiasis, breve revisión y recomendaciones. Revista Mexicana de Patología Clínica. 46(2):86-91. https://www.medigraphic.com/cgi-bin/new/resumen.cgi?IDARTICULO=2645 [ Links ]
LEROY J, Cornu M, Deleplancque AS, Loridant S, Dutoit E, Sendid B. 2017. Sushi, ceviche and gnathostomiasis - A case report and review of imported infections. Travel Medicine and Infectious Disease. 20(11): 26-30. https://doi.org/10.1016/j.tmaid.2017.10.010 [ Links ]
LIMA DOS SANTOS CAM, Howgate P. 2011. Fishborne zoonotic parasites and aquaculture: A review. Aquaculture. 318(3): 253-261. https://doi.org/10.1016/j.aquaculture.2011.05.046 [ Links ]
LOBNA SMA, Metawea YF, Elsheikha HM. 2010. Prevalence of heterophyiosis in Tilapia fish and humans in Northern Egypt. Parasitology Research. 107(4):1029-1034. https://doi.org/10.1007/s00436-010-1976-x [ Links ]
MADSEN H, Dung BT, The DT, Viet NK, Dalsgaard A, Van PT. 2015. The role of rice fields, fish ponds and water canals for transmission of fish-borne zoonotic trematodes in aquaculture ponds in Nam Dinh Province, Vietnam. Parasites and Vectors. 8(1):1-11. https://doi.org/10.1186/s13071-015-1237-z [ Links ]
MAHMOUD, MA, Abdelsalam M, Mahdy OA, El Miniawy HMF, Ahmed ZAM, Osman AH, Mohamed HMH, Khattab AM, Zaki Ewiss MA. 2016. Infectious bacterial pathogens, parasites and pathological correlations of sewage pollution as an important threat to farmed fishes in Egypt. Environmental Pollution. 219(1): 939-948. https://doi.org/10.1016/j.envpol.2016.09.044 [ Links ]
MJAKAKHAMIS H, Sagweorina P. 2017. Prevalence and diversity of internal cestode parasites infected Nile tilapia (Oreochromis niloticus) and african catfish (Clarias gariepinus) in farmers fresh water ponds in Kenya. American Scientific Research Journal for Engineering, Technology, and Sciences. 34(1):123-137. ISSN: 2313-4410. https://asrjetsjournal.org/index.php/American_Scientific_Journal/article/view/3076 [ Links ]
MORALES-SERNA FN, Chapa-López M, Martínez-Brown JM, Ibarra-Castro L, Medina- Guerrero RM, Fajer-Ávila EJ. 2018. Efficacy of praziquantel and a combination anthelmintic (Adecto®) in bath treatments against Tagia ecuadori and Neobenedenia melleni (Monogenea), parasites of bullseye puffer fish. Aquaculture. 492(11): 361-368. https://doi.org/10.1016/j.aquaculture.2018.04.043 [ Links ]
MUTENGU C, Mhlanga W, Mupangwa JF. 2018. Occurrence of Clinostomum metacercariae in Oreochromis mossambicus from Mashoko Dam, Masvingo Province, Zimbabwe. Scientifica. 2018(1): 1-6. https://doi.org/10.1155/2018/9565049 [ Links ]
NEGRETE L, Damborenea C. 2017. Phylum Platyhelminthes: Macroparásitos Diversidad y biología. La Plata, Argentina: Editorial de la Universidad Nacional de La Plata. Pp. 21- 35. ISBN: 978-950-34-1521-4. https://www.researchgate.net/publication/319693656_Phylum_Platyhelminthes [ Links ]
OJWALA RA, Otachi EO, Kitaka NK. 2018. Effect of water quality on the parasite assemblages infecting Nile tilapia in selected fish farms in Nakuru County, Kenya. Parasitology Research. 117(11):3459-3471. https://doi.org/10.1007/s00436-018-6042-0 [ Links ]
OKOYE IC, Abu SJ, Obiezue NNR, Ofoezie IE. 2014. Prevalence and seasonality of parasites of fish in Agulu Lake, Southeast, Nigeria. African Journal of Biotechnology. 13(3):502-508. https://doi.org/10.5897/ajb2013.13384 [ Links ]
OLOPADE OA, Taiwo IO, Lamidi AA, Awonaike OA. 2016. Proximate Composition of Nile Tilapia (Oreochromis niloticus) (Linnaeus, 1758) and Tilapia Hybrid (Red Tilapia) from Oyan Lake, Nigeria. Food Science and Technology. 73(1): 0-4. https://doi.org/10.15835/buasvmcn-fst:11973 [ Links ]
OTACHI E, Magana A, Jirsa F, Fellner-Frank C. 2014. Parasites of commercially important fish from Lake Naivasha, Rift Valley, Kenya. Parasitology Research. 113(3): 1057-1067. https://doi.org/10.1007/s00436-013-3741-4 [ Links ]
OTACHI E, Szostakowska B, Jirsa F, Fellner-Frank C. 2015. Parasite communities of the elongate tigerfish Hydrocynus forskahlii (Cuvier 1819) and redbelly tilapia Tilapia zillii (Gervais 1848) from Lake Turkana, Kenya: Influence of host sex and size. Acta Parasitologica. 60(1):9-20. https://doi.org/10.1515/ap-2015-0002 [ Links ]
PETNEY T, Andrews RH, Saijuntha W, Wenz-Mücke A, Sithithaworn P. 2013. The zoonotic, fish-borne liver flukes Clonorchis sinensis, Opisthorchis felineus and Opisthorchis viverrini. International Journal for Parasitology. 43(12-13):1031-1046. https://doi.org/10.1016/j.ijpara.2013.07.007 [ Links ]
PETNEY T, Taraschewski H. 2011. Waterborne parasitic diseases: Hydrology, Regional Development, and Control. Treatise on Water Science. 3(1):303-366. https://doi.org/10.1016/B978-0-444-53199-5.00061-0 [ Links ]
PINHEIRO RHDS, Furtado AP, Dos Santos JN, Giese EG. 2019. Contracaecum larvae: morphological and morphometric retrospective analysis, biogeography and zoonotic risk in the amazon. Revista Brasileira de Parasitologia Veterinaria. 28(1): 12-32. https://doi.org/10.1590/s1984-29612019002 [ Links ]
PINTO HA, Gonçalves NQ, López-Hernandez D, Pulido-Murillo EA, Melo AL. 2018. The life cycle of a zoonotic parasite reassessed: Experimental infection of Melanoides tuberculata (Mollusca: Thiaridae) with Centrocestus formosanus (Trematoda: Heterophyidae). PLoS ONE. 13(4):1-13. https://doi.org/10.1371/journal.pone.0194161 [ Links ]
PINTO HA, Mati VLT, Melo AL. 2014. Metacercarial infection of wild nile tilapia (Oreochromis niloticus) from Brazil. Scientific World Journal. 2014(19): 1-7. https://doi.org/10.1155/2014/807492 [ Links ]
PORTELA ACV, Silveira JGF, Damaceno MA, da Silva AFB, de Jesus RB, Pilarski F, Gadaj A, Mooney MH, Paschoal JAR. 2020. Food safety evaluation for the use of albendazole in fish: residual depletion profile and withdrawal period estimation. Food Additives and Contaminants - Part A Chemistry, Analysis, Control, Exposure and Risk Assessment. 37(4):596-606. https://doi.org/10.1080/19440049.2020.1719285 [ Links ]
PRITT B. 2015. Molecular diagnostics in the diagnosis of parasitic infection. In Methods in Microbiology. Minessota, USA: Elsevier Ltd. Pp. 160. ISBN: 978-0-12-803297-8. https://doi.org/10.1016/bs.mim.2015.05.001 [ Links ]
SALGADO-MALDONADO G, Aguilar-Aguilar R, Cabañas-Carranza G, Soto-Galera E, Mendoza-Palmero C. 2005. Helminth parasites in freshwater fish from the Papaloapan river basin, Mexico. Parasitology Research. 96(2): 69-89. https://doi.org/10.1007/s00436- 005-1315-9 [ Links ]
SALGADO-MALDONADO G, Caspeta-Mandujano JM, Moravec F, Soto-Galera E, Rodiles-Hernández R, Cabañas-Carranza G, Montoya-Mendoza J. 2011. Helminth parasites of freshwater fish in Chiapas, Mexico. Parasitology Research. 108(1): 31-59. https://doi.org/10.1007/s00436-010-2035-3 [ Links ]
SÈDOGBO MH, Zannou BT, Siko JE, Tossavi ND, Togla I, Fiogbé ED, Ibikounlé M. 2019. Faune des métazoaires parasites de Clarias gariepinus (Clariidae) et de Oreochromis niloticus (Cichlidae), deux poissons des whédos du delta supérieur du fleuve Ouémé au sud du Bénin. International Journal of Biological and Chemical Sciences. 13(2): 983-997. https://doi.org/10.4314/ijbcs.v13i2.33 [ Links ]
SEPULVEDA MS, Kinsella JM. 2013. Helminth collection and identification from wildlife.Journal of Visualized Experiments. 82(1):1-5. https://doi.org/10.3791/51000 [ Links ]
Servicio de información agroalimentaria y pesquera (SIAP). 2022. Panorama agroalimentario 2022. México. Pp. 217 https://www.gob.mx/siap/acciones-y- programas/produccion-pesquera [ Links ]
SITJÀ-BOBADILLA A, Oidtmann B. 2017. Integrated Pathogen Management Strategies in Fish Farming. In Fish Diseases: Prevention and Control Strategies. London, United Kingdom. Elsevier. Pp. 119-144. ISBN: 9780128045640 https://doi.org/10.1016/B978-0-12-804564-0.00005-3 [ Links ]
SOLER JIMÉNEZ L, Paredez Trujillo A, Vidal Martínez V. 2016. Helminth parasites of finfish commercial aquaculture in Latin America. Journal of Helminthology. 1(12): 1-27. https://doi.org/10.1017/S0022149X16000833 [ Links ]
TESANA S, Thabsripair P, Suwannatrai A, Haruay S, Piratae S, Khampoosa P, Thammasiri C, Prasopdee S, Kulsantiwong J, Chalorkpunrut P, Jones MK. 2014. Parasite surveys and environmental management for prevention of parasitic infection in cultivated Barbonymus gonionotus (Cyprinidae) in fishponds, in an opisthorchiasis endemic area of northeast Thailand. Aquaculture. 428(429): 54-60. https://doi.org/10.1016/j.aquaculture.2014.02.031 [ Links ]
VAN DE N, Le TH, Murrell KD. 2012. Prevalence and intensity of fish-borne zoonotic trematodes in cultured freshwater fish from rural and Urban Areas of Northern Vietnam. Journal of Parasitology. 98(5):1023-1025. https://doi.org/10.1645/GE-3112.1 [ Links ]
VAN PHAN T, Ersboll AK, Nguyen KV, Madsen H, Dalsgaard A. 2010. Farm-level risk factors for Fish-borne zoonotic trematode infection in integrated Small-scale fish farms in Northern Vietnam. PLoS Neglected Tropical Diseases. 4(7): 1-9. https://doi.org/10.1371/journal.pntd.0000742 [ Links ]
WALAKIRA J, Akoll P, Engole M, Sserwadda M, Nkambo M, Namulawa V, Kityo G. 2014. Common fish diseases and parasites affecting wild and farmed tilapia and catfish in central and western Uganda. Uganda Journal of Agricultural Sciences. 15(2): 113-125. ISSN: 1026-0919. https://www.ajol.info/index.php/ujas/article/view/126198 [ Links ]
WANG D, Young N, Korhonen P, Gasser R. 2018. Clonorchis sinensis and Clonorchiasis: The relevance of exploring genetic variation: Advances in Parasitology. London, United Kingdom: Elsevier. Pp. 155-208. Elsevier Ltd. ISBN: 978-0-12-815169-3 https://doi.org/10.1016/bs.apar.2018.03.006 [ Links ]
WANG ML, Chen HY, Shih HH. 2017a. Occurrence and distribution of yellow grub trematodes (Clinostomum complanatum) infection in Taiwan. Parasitology Research. 116(6):1761-1771. https://doi.org/10.1007/s00436-017-5457-3 [ Links ]
WANG M, Luo L, Chen X, Fang Y. 2017b. Investigation on sanitation of freshwater aquaculture environments and Clonorchis sinensis intermediate host infection in a city of Pearl River Delta region, China. Chin J Schisto Control. 29(6):716-719. https://doi:10.16250/j.32.1374.2017120 [ Links ]
WATTERSON A, Little D, Young JA, Murray F, Doi L, Boyd KA, Azim E. 2012. Scoping a Public health impact assessment of aquaculture with particular reference to tilapia in the UK. ISRN Public Health. 2012(1):1-18. https://doi.org/10.5402/2012/203796 [ Links ]
WILLIAMS M, Hernandez-Jover M, Shamsi S. 2020a. A critical appraisal of global testing protocols for zoonotic parasites in imported seafood applied to seafood safety in Australia. Foods. 9(4): 1-23. https://doi.org/10.3390/foods9040448 [ Links ]
WILLIAMS M, Hernandez-Jover M, Shamsi S. 2020b. Fish substitutions which may increase human health risks from zoonotic seafood borne parasites: A review. Food Control. 20(1):1-45. https://doi.org/10.1016/j.foodcont.2020.107429 [ Links ]
WIRIYA B, Clausen JH, Inpankaew T, Thaenkham U, Jittapalapong S, Satapornvanit K, Dalsgaard A. 2013. Fish-borne trematodes in cultured Nile tilapia (Oreochromis niloticus) and wild-caught fish from Thailand. Veterinary Parasitology. 198(1): 230-234. https://doi.org/10.1016/j.vetpar.2013.08.008 [ Links ]
ZHAO-RONG L, Robin G, De-hua L, An-xing L, Xing-quan Z, Xing-bing Y, Yue-yi F. 2005. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 1(1): 31-41. https://doi.org/10.1016/S1473-3099(04)01252-6 [ Links ]
Received: June 07, 2021; Accepted: February 25, 2023











 texto en
texto en