Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Abanico veterinario
versão On-line ISSN 2448-6132versão impressa ISSN 2007-428X
Abanico vet vol.13 Tepic Jan./Dez. 2023 Epub 09-Jun-2023
https://doi.org/10.21929/abavet2023.6
Estudio de Caso
Frequency of Campylobacter fetus in bulls in the central zone of Tamaulipas Mexico
1Facultad de Medicina Veterinaria y Zootecnia “Dr. Norberto Treviño Zapata”. Universidad Autónoma de Tamaulipas. Ciudad Victoria, Tamaulipas, México.
Bovine Genital Campylobacteriosis (BGC) is a contagious infectious disease that affects cattle. The disease is considered obligatory reporting and it is included in list B of terrestrial animal diseases according to the World Organization for Animal Health (WOAH). In ruminants, Campylobacter fetus subsp. fetus (Cff) has been shown to affect the enteric system, especially the intestine, and it is one of the main causes of infertility and abortion in cattle, sheep and goats. It has been reported in several countries around the world. In Mexico, the presence of this pathogen has not been studied, so the objective of this research was to detect Campylobacter fetus subsp. veneralis (CFv) by the PCR technique, and to perform its genetic characterization in bulls from the central area of Tamaulipas, Mexico. This is the first report of this pathogen by molecular methods in this country.
Keywords: Campylobacter fetus veneralis; bulls; sperm; preputial lavage; Mexico
La Campilobacteriosis Genital Bovina (CGB) es una enfermedad infecto contagiosa que afecta al ganado bovino. La enfermedad es considerada de reporte obligatorio y está incluida en la lista B de enfermedades de los animales terrestres según la Organización Mundial de Salud Animal (OMSA). En rumiantes Campylobacter fetus subsp. fetus se ha demostrado que afecta el sistema entérico, especialmente el intestino y es una de las principales causas de infertilidad y aborto en bovinos, ovinos y caprinos. Se ha reportado en varios países del mundo. En México poco se ha estudiado la presencia de este patógeno; en este estudio se comprobó la existencia de Campylobacter fetus subsp. veneralis en toros de la zona centro de Tamaulipas México por la técnica de PCR y secuenciación del producto amplificado, por lo que representa el primer reporte de este patógeno por métodos moleculares en este país.
Palabras claves: Campylobacter fetus veneralis; toros; semen; lavado prepucial; México
INTRODUCTION
Bovine genital campylobacteriosis (BGC) is an infectious disease affecting cattle. The Campylobacter genus is responsible for this disease. They are Gram-negative, spiral- shaped, motile bacteria. Currently, 32 Campylobacter species are recognized (Chukwu et al., 2019) which Campylobacter fetus subsp. fetus (Cff) and Campylobacter fetus subsp. venerealis (Cfv) affect the reproductive system of cattle (Chiapparrone et al., 2014). The disease is considered a mandatory reportable disease, included in List B of terrestrial animal diseases according to the World Organization for Animal Health (WOAH), formerly OIE (Tshipamba et al., 2020). In ruminants Cff has been shown that the pathogen affects the enteric system, especially the intestine (Li et al., 2022), on the other hand, bulls with Cfv are reservoir because the bacteria live and adapt in preputial cysts, while in cows colonizations are in vagina, cervix and uterus ((Cagnoli et al., 2020 and Lúcio et al., 2019) inducing proinflammatory processes (Campos-Múzquez et al., 2021) due to internalization in cells of the endometrial epithelium (Campos-Múzquez et al., 2019) causing abortions (Clune et al., 2022); C. fetus can also affect humans and has been reported to induce systemic infections (Adhikari et al., 2022) endocarditis (Lynch et al., 2022) and meningitis (Fernández et al., 2022). Bacteriological isolation and the use of biochemical tests play an important role for the identification of C. fetus and differentiation between Cff and Cfv, as they are considered the gold standard, however, the implementation of these culture techniques is difficult, as Campylobacter requires more demanding in vitro culture conditions than other bacterial genera, in addition, the culture is poor in reliability and reproducibility (Iraola et al., 2016). Different methods can be used for Cfv identification, such as antigen-antibody reaction as Enzyme-Linked Immunoassays (ELISA), Direct Immunofluorescence (DI) (Dorsch et al., 2022) and molecular methods as Pulsed Field Gel Electrophoresis (PFGE), Amplified Fragment Length Polymorphism (AFLP), multilocus sequence typing and Polymerase Chain Reaction (PCR). Currently PCR is the test with the most promising alternative to efficiently detect Cfv from field samples (Chaban et al., 2012). The objective of this study was to detect the presence of C. fetus by PCR in smegma from bulls used as breeders in the central zone of Tamaulipas state.
MATERIAL AND METHODS
Sampling
A convenience sampling was carried out in bovine stallions older than 2 years, of different breeds (Charolais, Beefmaster, red Brangus, Brahman) in Cattle Production Units (CPU) located in the central zone of Tamaulipas, Mexico. The study was carried out from August to December 2020. To obtain preputial samples, sterile disposable syringes and cannulas were used to deposit 60 mL of Ringer's Solution (PISA®) in the foreskin of each stallion; then the preputial orifice was closed and a vigorous massage was performed for 5 minutes throughout the foreskin, subsequently the contents of the lavage (smegma) were obtained by draining and deposited in a sterile plastic bag with a hermetic seal. The semen samples were obtained by electroejaculation using minitube® equipment. The stimulation of the ejaculate did not exceed 20 volts per animal. For the collection of the ejaculate, a collection case was used, which holds a latex funnel, at the end of which a graduated tube was placed to receive the semen; this tube was identified before being used. The samples were kept refrigerated (4 °C) during transport and until processing (Silveira et al., 2018).
DNA isolation
The collected samples were centrifuged at 604 xG for 5 min (Centurion Sc limited, UK) in sterile 50 ml plastic tubes, then the supernatant was discarded and the sediment was reconstituted with 0.2 ml of sterile 1X PBS. For genomic DNA isolation, all samples were treated with a commercial DNeasy blood and tissue kit (QIAGEN® Germany), following the manufacturer's instructions. Once the DNA collection process was completed, the genomic products were kept frozen (-20 ˚C) until further use. To evaluate DNA purification, a spectrophotometric study was performed at 260/280 nanometers (JENWAY Genova, UK), and ideal purity values were considered to be 1.8 to 2.0; DNA samples found to be in the range were subjected to DNA amplification by PCR.
DNA amplification and electrophoresis
A multiplex PCR was used; the primers used were nC1165g4F (AGGACACACAAATGGTAACTGG) and nC1165g4R (GATTGTATATAGCGACTTTGC) to detect a region of the Cfv cstA gene with amplification products of 233 base pairs (bp); primers MG3F (GGTAGCCGCAGCTAAGAT) and MG4R (TAGCTACACAATAACGACAAC) detect the virB11 Cff gene region amplifying to 764bp (Iraola et al., 2012 y Hum et al., 1997). As a positive control for the PCR reaction, nucleic acid from an ATCC reference strain of Cff 27374 was used, subsequently when performing the PCR assays, a sample (sample 1) with amplification suggestive of Cfv was identified. The amplification result was sequenced by the Institute of Biotechnology of UNAM. The sequence was analyzed in the GenBank database using the BLAST online tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The PCR reaction was performed in 50 µL, (containing 5 µL of 10 X Buffer (Tris-HCl 100 mM pH 8.4, KCl 500 mM, gelatin 10 µg/ml, BSA 1.5 mg/ml, 1% Triton X 100 (Biotecmol, BIOTECMOL®, CDMX Mexico), dNTPS 0. 2 mM (InvitrogenTM, Life technologies; USA), 25 µM of each primer, MgCl2 3.0 mM (BIOTECMOL® CDMX, Mexico), 5 U of Taq DNA polymerase (Amplificasa® BIOTECMOL, CDMX, Mexico), plus 300 ng of DNA, finally adjusted with nuclease-free water to 50 µL. The PCR cycling conditions used were initial denaturation for 7 min at 95 °C followed by 35 cycles of denaturation for 30 sec at 94 °C, alignment 30 sec at 53 °C; and extension for 1 min at 72 °C, and a final extension of 2 min at 72 °C (Iraola et al., 2012). The final products were analyzed by 3% agarose gel electrophoresis, and then stained with ethidium bromide (promega) at a concentration of 0.5 µg/mL for 30 min. For visualization and identification of PCR products, a UV transilluminator was used and photodocumented on the E-gel imaging system. PCR products were purified with the commercial PureLink™ PCR Purification Kit (Invitrogen, Carlsbad, USA). Some PCR products were sequenced at the Institute of Biotechnology UNAM, Mexico City, Mexico. Sequencing results were analyzed in BLASTn. For sequence comparison, the NCBI/GenBank database was used (Altschul et al., 2012).
RESULTS
Thirty-eight bulls were sampled; one sample was obtained from each; 31 from preputial lavage and 7 from semen. 18.4% were positive samples (7/38) (Figure 1), of these, two were from semen and five from preputial lavage. Of the 7 positive samples, 5 PCR products were subjected to sequencing. All sequences were compared considering the bacterium with accession number JF901335.1 in Genbank.

Figure 1 2.5% agarose gel with the PCR products. Lane M: 100 mpb (BIOLINE, USA); lanes 1-9: Cfv positive; lane 8: negative control; lane 9: positive control Cff 764 bp and Cfv 233 bp
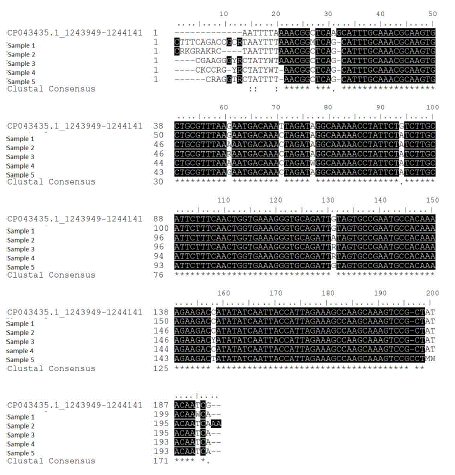
The nucleotide sequences of the amplicons obtained from field samples by PCR were identical to those found in the GenBank database. The analysis showed between 99.17 and 97.87% identity with other Cfv (Fig. 2). These results demonstrated that Cfv is present in herds in central Tamaulipas, and that Cfv plays an important role in the reproductive health of cattle.
DISCUSSION
It is documented that BGC affects cattle herds; pathology caused by Campylobacter fetus (C. fetus) subspecies venerealis (Cfv). BGC is one of the main causes of infertility and abortion in cattle, sheep and goats; and its presence has been confirmed in several countries of the American continent such as Argentina, Brazil, Costa Rica, Jamaica and Mexico. However, in Mexico, this pathogen has been little studied in livestock areas. The detection of this pathogenic subspecies in bulls used as sires is of utmost importance for the control of this disease in herds, since they are asymptomatic carriers; and, in addition to being used for direct mating, semen is frequently used for artificial insemination, both fresh and in straws preserved in liquid nitrogen. In addition to infertility problems, Cff is considered the main cause of sperm alterations due to the ability of these bacteria to adhere to the spermatozoa causing damage to the acrosomal membrane as well as to the sperm chromatin, which decreases semen quality and consequently the pregnancy percentage, besides influencing infertility in females due to the transmission of bacteria to the genital tract during natural mating (Cagnoli et al., 2020).
The diagnosis of this disease can be performed by various methods such as bacteriological isolation, serological methods such as ELISA and DI, and molecular assays such as PCR (Mshelia et al., 2010; Clune et al., 2022). Bacteriological isolation is the least sensitive method, as demonstrated by Groff et al., en 2010, where they compared this method with PCR, resulting in this molecular method being 8.5 times more sensitive than traditional bacteriological isolation. In this study, PCR was used following the recommendations of other researchers; for the identification of Cfv with the cstA gene and for Cff with the virB11 gene (Hum et al., 1997; Iraola et al., 2012), given that the primers generated for the identification of these genes can detect specific DNA sequences of Cfv and Cff; with the PCR developed allowed positive cases of CGB to be detected in the study area. Another method that has been used is DI; however, Ferreira en 2002 shows that the technique does not distinguish C. fetus subspecies.
In Mexico, beef cattle have been exported to the United States of America since 1994, and the state of Tamaulipas has been characterized for having one of the largest inventories in the country. In the 2020-2021 cycle, the export of live cattle was 30,896 head to the United States according to data from the Secretariat of Agriculture and Rural Development (SADER). Considering the need to increase the number of animals to supply the demand, it is necessary to have an animal health diagnosis of diseases that affect reproductive parameters. The success of the diagnosis depends largely on sampling; based on previous reports reported by Silveira et al., 2018 and Delpiazzo et al., 2021.
The prevalence of BGC is variable in different areas of the world, and there are several factors that favor its transmission (Hoque et al., 2021). In Mexico, little has been reported on the presence of Campylobacter in cattle; the only study found in our country was conducted by Barajas en 2013 in tropical areas of Mexico; by means of ELISA test, it revealed a seroprevalence of 21.3% in cattle. The detection method was different and the sampling was lower; the frequency detected in this study was 18.4%, indicating that BGC is present in Tamaulipas cattle herds. It is worth mentioning that Barajas' work was carried out with cattle from the humid tropics of the extension program for cattle ranchers in northern Veracruz, a state adjacent to Tamaulipas and with climatic conditions similar to the study area.
The objective of this study was to detect the presence of C. fetus in bulls from the central zone of Tamaulipas state. From the 7 amplifications suggestive of Cfv by PCR, 5 were evaluated by sequencing. The results obtained in the bioinformatic analysis confirm that the amplification corresponds to a region of the virB11 gene which is a sequence of pathogenicity islands present only in Cfv with 233 bp and absent in Cff, which allows differentiating these two subspecies by molecular method. The results obtained in the present work are consistent with what was previously published by Iraola et al., 2016, so the epidemiological situation could be at risk due to the spread of this pathogen by the use of carrier bulls for reproductive purposes.
CONCLUSION
In this study, the presence of Campylobacter fetus subsp. venerealis was detected by PCR and corroborated by sequencing with a frequency of 18.4% of the bulls analyzed from the central zone of Tamaulipas state, which represents the first report in Mexico of this bacterium by molecular methods.
ACKNOWLEDGMENTS
To ZVD. Francisco Trejo Meza from The Tamaulipas Regional Livestock Association's Genetic Improvement Center for allowing the sampling of milking bulls.
LITERATURA CITADA
ADHIKARI P, Antala D, Bhandari B, Mohamed K, Egoryan G, Stake JJ, Friedman H. 2022. A Case of Campylobacter Fetus Subspecies Fetus Systemic Infection. Cureus Journal of Medical Science. 14:e23963. https://doi.org/10.7759/cureus.23963 [ Links ]
ALTSCHUL SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. Journal of Molecular Biology. 215:403-10. https://doi.org/10.1016/S0022- 2836(05)80360-2 [ Links ]
BARAJAS JA. 2013. Seroepidemiología de Campylobacter fetus subes. venerealis en ganado bovino en el trópico de México. Congreso Nacional de Buiatría XXXVII. https://www.expresionesveterinarias.com/2018/09/seroepidemiologia-de- campylobacter.html [ Links ]
CAGNOLI CI, Chiapparrone ML, Cacciato CS, Rodríguez MG, Aller JF, Catena MDC. 2020. Effects of Campylobacter fetus on bull sperm quality. Microbial Pathogenesis. 149:1-5. https://doi.org/10.1016/j.micpath.2020.104486 [ Links ]
CAMPOS-MÚZQUEZ LG, Méndez ET, Arellano B, Martínez D. 2019. Campylobacter fetus is Internalized by Bovine Endometrial Epithelial Cells. Polish Journal of Microbiology. 68:217-224. https://doi.org/10.33073/pjm-2019-022 [ Links ]
CAMPOS-MÚZQUEZ LG, Méndez ET, Palacios N, Martínez D. 2021. Campylobacter fetus Induced Proinflammatory Response in Bovine Endometrial Epithelial Cells. Polish Journal of Microbiology. 70:99-106. https://doi.org/10.33073/pjm-2021-009 [ Links ]
CHABAN B, Chu S, Hendrick S, Waldner C, Hill JE. 2012. Evaluation of a Campylobacter fetus subspecies venerealis real-time quantitative polymerase chain reaction for direct analysis of bovine preputial samples. Canadian Journal of Veterinary Research. 76:166-73. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3384278/ [ Links ]
CHIAPPARRONE ML, Morán PE, Echevarría HM, Soto P, Paolicchi FA, Catena M. 2014. Campylobacter fetus subsp. venerealis adhesion to MDBK cells. Revista Argentina de Microbiología. 46:269-70. https://doi.org/10.1016/S0325-7541(14)70081-1 [ Links ]
CHUKWU MO, Luther KA, Ubomba-Jaswa E, Obi L, Dewar JB. 2019. Characterization and Phylogenetic Analysis of Campylobacter Species Isolated from Paediatric Stool and Water Samples in the Northwest Province, South Africa. International Journal of Environmental Research and Public Health. 16:2205. https://doi.org/10.3390/ijerph16122205 [ Links ]
CLUNE T, Bruce M, Glanville E, Campbeñ AS, Lockwood A, Hancock S, Thompson AN, Beetson S, Brookes D, Trengove C. 2022. Seropositivity to Campylobacter and association with abortion and lamb mortality in maiden ewes from Western Australia, South Australia and Victoria. Australian Veterinary Journal. 10.1111/avj.13173. Advance online publication. https://doi.org/10.1111/avj.13173 [ Links ]
DELPIAZZO R, Barcellos M, Barros S, Betancor L, Fraga M, Gil J, Iraola G, Morsella C, Paolicchi F, Pérez R, Riet-Correa F, Sanguinetti M, Silva A, Silva S, Calleros L. 2021. Accurate and fast identification of Campylobacter fetus in bulls by real-time PCR targeting a 16S rRNA gene sequence. Veterinary and Animal Science. 11:100163. https://doi.org/10.1016/j.vas.2020.100163 [ Links ]
DORSCH MA, Francia ME, Tana LR, González C, Cabrera A, Calleros L, Sanguinetti M, Barcellos M, Zarantonelli L, Ciuffo C, Maya L, Castells M, Mirazo S, Silva S, Rabaza A, Caffarena RD, Doncel B, Aráoz V, Matto C, Armendano I, Giannitti F. 2022. Diagnostic Investigation of 100 Cases of Abortion in Sheep in Uruguay: 2015-2021. Frontiers in Veterinary Science. 9:904786. https://doi.org/10.3389/fvets.2022.904786 [ Links ]
FERNÁNDEZ R, Lorenzo-Vizcaya AM, Bustillo M, Fernández R. 2022. Campylobacter fetus meningitis and subdural empyema. Enfermedades infecciosas y microbiologia clinica (English ed.), 40:212-213. https://doi.org/10.1016/j.eimce.2022.02.005 [ Links ]
FERREIRA F, Oliveira P, Bastos F, Paula M, Leite M, Pereira L. 2002. Evaluation of direct fluorescent antibody test for the diagnosis of bovine genital campylobacteriosis. Rev Latinoam Microbiol. 44:118-23. https://pubmed.ncbi.nlm.nih.gov/17063594/ [ Links ]
GROFF A, Kirinus J, Sáe SM, Manchado G, Mateus MC, Agueda V. 2010. Polymerase chain reaction for the diagnosis of bovine genital campylobacteriosis. Pesquisa Veterinaria Brasileira. 30:1031-1035. https://doi.org/10.1590/S0100- 736X2010001200005 [ Links ]
HOQUE N, Islam SK, Uddin MN, Arif M, Haque AK, Neogi SB, Hossain MM, Yamasaki S, Kabir SM. 2021. Prevalence, Risk Factors, and Molecular Detection of Campylobacter in Farmed Cattle of Selected Districts in Bangladesh. Pathogens. 10:313. https://doi.org/10.3390/pathogens10030313 [ Links ]
HUM S, Quinn K, Brunner J, Slw O. 1997 Evaluation of a PCR assay for identification and differentiation of Campylobacter fetus subspecies. Australian Veterinary Journal. 75:827- 831. https://doi.org/10.1111/j.1751-0813.1997.tb15665.x [ Links ]
IRAOLA G, Hernández M, Calleros L, Paolicchi F, Silveyra S, Velilla A, Carretto L, Rodríguez E, Pérez R. 2012. Application of a multiplex PCR assay for Campylobacter fetus detection and subspecies differentiation in uncultured samples of aborted bovine fetuses. Journal of Veterinary Science. 13:371-6 https://doi.org/10.4142/jvs.2012.13.4.371 [ Links ]
IRAOLA G, Pérez R, Betancor L, Marandino A, Morsella C, Méndez A, Paolicchi F, Piccirillo A, Tomás G, Velilla A, Calleros L. 2016. A novel real-time PCR assay for quantitative detection of Campylobacter fetus based on ribosomal sequences. BMC Veterinary Research. Dec12:286. https://doi.org/10.1186/s12917-016-0913-3 [ Links ]
LI X, Tang H, Xu Z, Tang H, Fan Z, Jiao X, Huang J. 2022. Prevalence and characteristics of Campylobacter from the genital tract of primates and ruminants in Eastern China. Transboundary and Emerging Diseases, 10.1111/tbed.14524. Advance online publication. https://doi.org/10.1111/tbed.14524 [ Links ]
LÚCIO ÉC, Barros MR, Mota RA, de Cássia Carvalho Maia R, Pinheiro JW. 2019. Identification of Campylobacter fetus subsp. venerealis virulence genes in cervical mucus from cows. Brazilian Journal of Microbiology. 50:1133-1137. https://doi.org/10.1007/s42770-019-00127-w [ Links ]
LYNCH CT, Buttimer C, Epping L, O'connor J, Walsh N, McCarthy C, O'brien D, Vaughan C, Semmler T, Bolton D, Coffey A, Lucey B. 2022. Phenotypic and genetic analyses of two Campylobacter fetus isolates from a patient with relapsed prosthetic valve endocarditis. Pathogens and Disease. 79:ftab055. https://doi.org/10.1093/femspd/ftab055 [ Links ]
MSHELIA GD, Amin JD, Egwu GO, Woldehiwet Z, Murray RD. 2012. The prevalence of bovine venereal campylobacteriosis in cattle herds in the Lake Chad basin of Nigeria. Tropical Animal Health and Production. 44:1487-1489. https://doi.org/10.1007/s11250- 012-0092-6 [ Links ]
SILVEIRA CDS, Fraga M, Giannitti F, Macías-Rioseco M, Riet-Correa F. 2018. Diagnosis of Bovine Genital Campylobacteriosis in South America. Frontiers in Veterinary Science. 5:321. https://doi.org/10.3389/fvets.2018.00321 [ Links ]
TSHIPAMBA ME, Akinola SA, Ngoma L, Mwanza M. 2020. Genome Sequence of Campylobacter fetus subsp. venerealis NW_ED23, Isolated from Bovine Sheath Wash. Microbiology Resource Announcements. 9:e00854-20. https://doi.org/10.1128/MRA.00854-20 [ Links ]
Received: August 01, 2022; Accepted: February 28, 2023











 texto em
texto em