Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Abanico veterinario
versión On-line ISSN 2448-6132versión impresa ISSN 2007-428X
Abanico vet vol.12 Tepic ene./dic. 2022 Epub 23-Jun-2023
https://doi.org/10.21929/abavet2022.15
Original article
Effect of coumestrol on the epididymis of adult dogs
1Doctorado en Ciencias de la Producción y de la Salud Animal. Universidad Nacional Autónoma de México.
2Departamento de Producción Agrícola y Animal, Universidad Autónoma Metropolitana Unidad Xochimilco.
3Unidad de Investigación en Inmunología, Instituto Mexicano del Seguro Social.
4Departamento de Morfología. Facultad de Medicina Veterinaria y Zootecnia. Universidad Nacional Autónoma de México.
The concern of canine overpopulation is related to zoonoses such as rabies, which is responsible for 99% of human rabies cases, causing the death of approximately 59,000 people per year. Surgical sterilization is an effective, costly and invasive control method with limited impact. The effects of phytoestrogens on reproductive activity have been commonly studied in females and a limited number in males. Therefore, the objective of this study was to know the effect of subcutaneous administration of coumestrol on the gonadal activity of adult dogs as an alternative for their reproductive control. Basic seminal evaluation parameters, epididymal structure by ultrasonography, histological characteristics, as well as the presence of coumestrol by fluorescence and serum levels of testosterone and estrogens were determined. The administration of coumestrol for five weeks reduced sperm production, and evidenced changes in the echodensity and cellularity of the epididymis, associated with serum concentrations of estradiol and testosterone. Therefore, it is concluded that coumestrol administered subcutaneously has an estrogenic effect that can be used as a non-invasive method to help control fertility in adult dogs.
Keywords: epididymis; spermatozoa; estrogens; phytoestrogens; testis; dog; reproductive control
La preocupación de la sobrepoblación canina se relaciona con zoonosis como la rabia, la cual es responsable del 99% de los casos de rabia humana, causante de la muerte de aproximadamente 59,000 personas al año. La esterilización quirúrgica es un método de control eficaz, costoso e invasivo siendo su impacto limitado. Los efectos de los fitoestrógenos en la actividad reproductiva se han estudiado comúnmente en hembras, y un número limitado en machos. Por lo anterior, el objetivo de este estudio fue conocer el efecto de la administración subcutánea de coumestrol en la actividad gonadal de perros adultos como alternativa para su control reproductivo. Se determinaron los parámetros de evaluación seminal básica, la estructura del epidídimo mediante ecografía, características histológicas, así como la presencia del coumestrol mediante fluorescencia y los niveles séricos de testosterona y estrógenos. La administración de coumestrol durante cinco semanas redujo la producción espermática, y evidenció cambios en la eco densidad y celularidad del epidídimo, asociados a las concentraciones séricas de estradiol y testosterona. Por lo que, se concluye que el coumestrol administrado vía subcutánea tiene un efecto estrogénico que puede utilizarse como un método no invasivo para ayudar a controlar la fertilidad de perros adultos.
Palabras clave: epidídimo; espermatozoides; estrógenos; fitoestrógenos; testículo; perro; control reproductivo
INTRODUCTION
Dog overpopulation is a worldwide problem which influences public and animal health (Rubel & Carbajo, 2019), because they are a source of health, political, socioeconomic and welfare problems, particularly in developing countries. These problems include roaming the streets causing traffic accidents, barking, aggression, and biting. The growing population of stray dogs in Latin American countries is alarming, several factors contribute to the increase in their population, the most common being abandonment by the responsible parties, which is the main cause of the number of dogs roaming streets or living in dog shelters (Mota-Rojas et al., 2021).
The main concern of dog overpopulation is related to zoonotic diseases, especially canine rabies, an underreported and neglected disease in developing countries, which is responsible for 99% of dog-transmitted human rabies cases worldwide, killing approximately 59,000 [95% confidence interval: 25,000-159,200] people worldwide annually. Efforts are underway to reduce human deaths due to dog-mediated rabies to zero by 2030 (Hampson et al., 2015; Hampson et al., 2019).
Surgical sterilization is the best method to prevent reproduction and, consequently, dog overpopulation. However, the impact of sterilization programs has been limited, mainly due to high operational costs that make it difficult to reach as many dogs as possible (Evans et al., 2022; Belsare & Vanak, 2020). Because of this, mass slaughter continues to be used as the main method of population control in much of the world, being little accepted by society, so the World Health Organization published guidelines in 1990 that discourage the use of slaughter and recommend alternative methods such as contraceptive methods (Smith et al., 2019).
Because of this, different research has focused on finding and proposing canine fertility control alternatives. It includes the use of synthetic hormones (e.g., synthetic androgens), chemical sterilization (e.g., zinc gluconate neutralized with arginine, calcium chloride), immunocontraception (e.g., gonadotropin hormone inhibitor vaccine) (Sandam et al., 2021; Root Kustritz, 2018; Asa, 2018; Massei et al., 2013), as well as, the use of therapeutic ultrasound at different frequencies (Leoci et al., 2015), among others. However, some methodologies may present different adverse effects and have not been shown to be fully effective (Sandam et al., 2021; Asa, 2018; Root Kustritz, 2018). For such reason, as a complementary method, the use of phytoestrogens is observed.
Phytoestrogens (PE) are plant-derived polyphenolic compounds, which are divided into several groups, including isoflavones (genistein), lignans (enterolactone), flavones (luteolin), stilbenes (resveratrol) and coumestanes (coumestrol) (Adler et al., 2015; Pérez- Rivero et al., 2009). They have similar structures to endogenous estrogens and can induce or inhibit the response in hormone receptors because they have the ability to bind to estrogen receptors (ER) alpha (ERα) and beta (ERβ) located in tissues in different organs including the reproductive. This binding is with higher affinity to ERβ (Rietjens et al., 2017).
Some research indicates that PEs act as endocrine disruptors by competing with endogenous estrogens for binding to their receptors and can produce positive or negative biological effects on animal health (Beszterda & Frański, 2018). Similar to what happens with any drug, it depends on the dose or route of administration of PEs the intensity of the effect on the target tissue, because they are subjected to different processes of absorption, biotransformation, distribution and excretion. In addition, their effects may vary depending on the phytoestrogen, species exposed, sex, route of administration, dose and duration of exposure, as well as the time of exposure during reproductive development or adult life. Thus, EFs have the ability to act as estrogenic agonists producing classical estrogenic effects or as estrogenic antagonists (inhibiting the effect of estrogens) (Domínguez-López et al., 2020; Mostrom & Evans, 2018).
Additionally, PEs can modulate the hypothalamus-pituitary axis, they also have the ability to inhibit aromatase (cytochrome P450) mRNA expression, producing the decrease of local estrogen biosynthesis, resulting in cellular inhibition, including spermatogenesis, participating as intracellular regulators of cell cycle and apoptosis (Domínguez-López et al., 2020; Lephart, 2015; Cerendolo et al., 2009; Kawakami et al., 2004).
Coumestrol (COU) is present in alfalfa (Medicago sativa), white clover (Trifolium repens), spinach (Spinacia oleracea) and soybean (Glycine max) sprouts (Cerendolo et al., 2009; Domínguez-López et al., 2020). It has the ability to act as an endocrine disruptor of ERα and Erβ, which are widely distributed in the male reproductive tract during development and adulthood (Cooke et al., 2021). In the dog, the presence of ER has been detected in interstitial Leydig cells, round spermatids, spermatogonia, and in the connective tissue of the epididymis (Serrano et al., 2008).
A peculiarity of COU is that it can be naturally detected by fluorescence when bound to ER, it is excited at a wavelength of 390-400 nanometers (blue) and emits fluorescence with a wavelength of 532-559 nanometers (green color) (Wang et al., 2014).
COU has been studied as a possible method of reproductive control in dogs. In females when administered orally, it induces a decrease in progesterone levels and an increase in serum estrogen values, without affecting the cellularity of the vaginal epithelium (Peña- Corona et al., 2020). In males, when administered orally, it affects the histoarchitecture of the germinal epithelium of the seminiferous tubes (Pérez-Rivero et al., 2009). The same effect has also been reported in hematophagous bats (Desmodus rotundus) males (Pérez-Rivero et al., 2014).
Spermatozoa that pass from the testes to the epididymis are non-functional gametes. It is during their transit through the epididymis where, through their interaction with proteins, they mature, acquire progressive motility and the ability to fertilize eggs (Ali Hassan et al., 2021; Cornwall, 2009), so the epididymis can be a target for the reproductive control of species considered to be disease transmitters. Due to the above, the aim of this study was to know the effect of subcutaneous administration of coumestrol in the epididymis of adult dogs as an alternative for their reproductive control.
MATERIAL AND METHODS
Animal Welfare
The study was approved with protocol number DC-2017/2-4, by the Internal Committee for the Care and Use of Animals from Veterinary Medicine and Zootechnics Faculty of the National Autonomous University of Mexico. It was performed with adherence to the principles of the 3 R's of research with animals (NCRRAR).
Animals
Ten male dogs between 2 and 3 years old, of different breeds, sexually mature, with an average weight of 14±2.9 kg, were included. Clinically healthy with both testes scrotalized.
Experiment
They were randomly divided into two groups with five dogs each: Control (C) and treatment with coumestrol (TCOU), and followed for 5 continuous weeks. In the first week, the TCOU group received a single dose of 1.2 mg/kg subcutaneously of coumestrol (COU, Sigma Aldrich® Lot# BCBH0742V Pcode: 101171142 and Lot# BCBN3969V Pcode: 101652427), dissolved in dimethyl sulfoxide (DMSO, Sigma Chemical Co®) to have a COU concentration: 100 µg/µl, which was mixed with 300 µl chitosan vehicle. Group C only received the DMSO and chitosan vehicle in the same amount and route of administration (Miranda-Castro & Lizarraga-Paulin, 2012).
Clinical studies
Each dog in both groups: C and TCOU, prior to treatment administration, at the first week of treatment administration and subsequently every week for 4 occasions, underwent four evaluations: evaluation of seminal production and quality, ultrasound to evaluate testes and epididymis, and blood collection for determination of hormonal indicators.
Sperm concentration, morphology and motility
After obtaining the ejaculate, progressive motility was evaluated at 4X and 10X, sperm concentration and morphology were determined according to the methodology proposed by Zuvela & Matson (2020) and Johnston (1991).
Ultrasonography
Dogs were placed in center lateral decubitus without sedation, to perform ultrasound evaluation of both testes and epididymis in a transscrotal manner. A K10-Vet ultrasound equipment (KontroLab®) and a 7.5 MHz microconvex transducer were used to quantitatively determine the echogenicity of the testicles, mediastinum and the head of the epididymis. From each anatomical region, digital images were obtained and evaluated in the sagittal plane, twenty areas were randomly selected on each image to determine the gray scale pixels of each one. A value of "0" totally black and "255" totally white was considered. ImageJ software (NIH; Morales et al., 2021) was used.
Gonadal morphology
In the fifth week, all dogs in both groups underwent bilateral orchiectomy under inhalation anesthesia (isoflurane-oxygen) and epididymal dissection of both testes was performed. Sections of epididymal head and body were fixed in Bouin's solution for processing by kerosene embedding and 3-micrometer thick sections were obtained and stained with hematoxylin and eosin (Aziz & Zeman-Pocrnich, 2022; Kurowicka et al., 2015).
Sections of the epididymal head were evaluated by light microscopy (Optisum® MIC 900T) microphotographs were taken at 20X and 40X. Subsequently, tubule diameter, area and thickness of the tubular epithelium, as well as, the area occupied by the sperm packet in the lumen of each tubule were determined with the LSM 5® (Carl Zeiss) computer program.
Identification of estrogen receptors
Two unstained histological sections of each testis from both groups (C and TCOU) were evaluated by Leica TCS SP2 confocal microscopy with a wavelength of 400 nanometers (blue light) for excitation and 550 nanometers to detect green fluorescence emission associated with COU bound to the estrogenic receptors present (Serrano et al., 2008; Pérez-Rivero et al., 2009). Digital images were also obtained to determine the intensity of the emitted fluorescence expressed in pixels with the ZEN Blue 3.5 Carl Zeiss Microscopy GmbH program.
Serum parameters of testosterone and estradiol
Blood samples were obtained by jugular venipuncture during daylight hours with at least 8 h of fasting, dispensed in tubes without anticoagulant. Serum testosterone and estradiol concentration was determined by enzyme-linked immunoadsorption assay (ELISA) using commercial kits (DRG Testosterone ELISA EIA-1559 and DRG Estradiol Sensitive ELISA EIA-4399, DRG Instruments GmbH®, Germany) and following the manufacturer's protocols. The analytical sensitivity of tests for testosterone and estradiol was 0.083 ng/ml and <1.399 pg/ml respectively. The intra-assay and inter-ensayo coefficients of variation of the tests were 3.59%, and 7.12% for testosterone, and of 6.36%, and 7.60% for estradiol. If the serum concentration was below the limit of quantification, that sample was assigned the lower limit value.
Statistical analysis
Statistical analysis was performed with PAST 3.01® software (Hammer, 2001). The medians ± standard error (SE) and the range between quartiles (Q1-Q3) were obtained for the cellularity parameters determined in gonads, as well as for the intensity of the ultrasound pixels and fluorescence images. Differences between groups were analyzed with the Mann-Whitney test. The significance level was set at p < 0.05.
RESULTS
Sperm production
The initial sperm concentration of group C was 1.79±0.001 X108 spermatozoa/ml, which in the following weeks did not show variations. On the contrary, in the TCOU group (Table 1), the sperm concentration in the ejaculates showed an evident numerical decrease from week one to four, the concentration determined in the fifth week which was 0.420±0.002X108 spermatozoa/ml was lower (p<0.05) than the concentrations determined in the previous four weeks.
Sperm motility decreased significantly (p<0.05) from week 3 onwards. Regarding sperm morphology, an increase was observed at week 3 of alterations in the tail and proximal and distal cytoplasmic droplets associated with maturation in the epididymis (Table 1).
Table 1 Weekly sperm concentration, morphology and motility of dogs treated with coumestrol (TCOU group)
|
Sperm concentration X108 spermatozoa/ml Median±SE (Q1-Q3) | ||||||
| Week | 1* | 2 | 3 | 4 | 5 | Mann- Whitney P |
| Treated | 1.79±0.001 (1.78-1.79) | 1.58±0.05 (1.48-1.68) | 1.17±0.09 (1.01-1.32) | 0.952±0.03 (0.41-1.49) | 0.420±0.002a (0.41-0.42) | <0.05 |
| Control | 1.79±0.001 (1.78-1.79) | 1.68±0.03 (1.68-1.78) | 1.68±0.04 (1.58-1.70) | 1.60±06 (1.55-1.79) | 1.78±0.02 (1.70-1.79) | >0.05 |
|
Sperm motility % Median (Q1-Q3) | ||||||
| Treated | >90 (89-92) | >90 (88-91) | 50a (50-50) | 50a (45-60) | 60a (50-60) | <0.05 |
| Control | >90 (89-92) | >90 (89-92) | >90 (89-92) | >90 (89-92) | >90(89-92) | >0.05 |
| Sperm morphology % ± SE | ||||||
| Treated Normal | 86±1.5 | 88±1.1 | 65±7.0a | 56±5.5a | 57±2.9a | <0.05 |
| Control Normal | 86±1.5 | 87±2.0 | 88±1.0 | 86±2.0 | 89±1.5 | >0.05 |
| Treated Tail rolled up | 1±0.80 | 4±1.5 | 10±4.5a | 9±4.5a | 5±2.5 | <0.05 |
| Control Tail rolled up | 1±0.80 | 1±0.5 | - | - | - | >0.05 |
| Treated Proximal drop | - | - | - | 2±2 | 7±4 | >0.05 |
| Control Proximal drop | - | - | - | - | - | - |
| Treated Distal drop | - | 1±0.5 | 1±0 | 7±1a | - | <0.05 |
| Control Distal drop | - | - | - | - | - | - |
| Treated Tail rolled up | 4±2 | 2±0.25 | 18±10 | 34±13a | 32±6.9a | <0.05 |
| Control Tail rolled up | 4±2 | 2±1 | - | - | 2±1 | >0.05 |
*Start of treatment. aIndicates the parameter where there is a significant difference in the Mann-Whitney test
Sonographic indicators and gonadal morphology
Gonadal echogenicity (Table 2), showed that the gonadal parenchyma of the TCOU group (69.9±2.9) was increased (p>0.05) with respect to the echogenicity determined in the C group (63.9±2.6).
Table 2 Gonadal echogenicity in control group and coumestrol-treated dogs
| Group/Quartile | Control Median±SE | Q1-Q3 Median | Coumestrol Median±SE | Q1-Q3 Median | Mann Whitney P |
|---|---|---|---|---|---|
| Parenchyma | 63.9±2.6 | 45.5-77.5 | 69.9±2.9 | 51.5-83.5 | >0.05 |
| Mediastinum | 67.3±2.0 | 55.5-77.0 | 60.0±2.4 | 46.5-73 | <0.01 |
| Epididymal head | 53±2.9 | 35.5-64 | 44±1.8 | 39-47 | <0.05 |
Gonadal ultrasound/Gray pixels
In testicular ultrasonography (Figure 1), pixel intensity showed a decrease in echogenicity in the mediastinum region (testicular network) and in the epididymal head region (p<0.05). The evaluation of cellularity in the epididymal head of both groups C and TCOU (Table 3) showed no differences between them; however, ranges of the values determined in each group observed in the quartiles were evident.
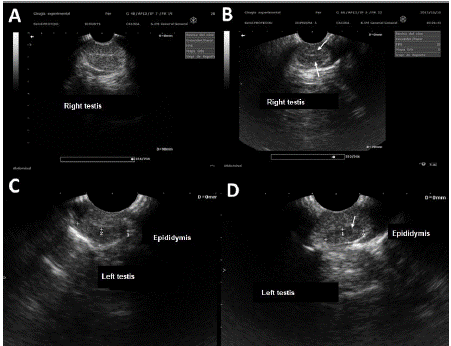
Figure 1 Ultrasound of the testis and epididymis. A. Control group. B. OCT group, note the decrease in echogenicity at the height of the testicular hilum (arrows). C. Evident echogenicity in epididymis of dogs in the control group. D. Lower echogenicity in epididymis of dogs in the TCOU group
Table 3 Cellularity parameters of the epididymis of dogs treated with coumestrol
| Epididymal head | Control Median±SE | Q1-Q3 Median | Coumestrol Median±SE | Q1-Q3 Median | Mann Whitney P |
|---|---|---|---|---|---|
| Morphometry | |||||
| Diameter µm | 273±5.7 | 235-306 | 314±5.3 | 279-340 | <0.01 |
| Area µm2 | 60882±2593 | 43665- 73608 | 79688±2716 | 61362- 90872 | <0.01 |
| Epithelium thickness µm | 59.7±1.0 | 53.4-64.4 | 75.9±1.0 | 67.0-83.6 | <0.01 |
| Length of microvilli in µm | 23.6±0.4 | 20.8-25.6 | 13.0±0.6 | 8.6-17.9 | <0.01 |
| Spermatozoa (Area of the sperm packet in µm2) | 12703.9±2026.7 | 7991.1- 18201.5 | 8371.8±2605.5 | 781.0- 14181.5 | <0.05 |
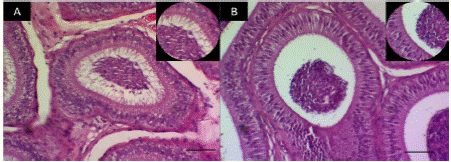
In the histological observations of epididymal cellularity (Figure 2), it is highlighted that the TCOU group presented a decrease in sperm mass and luminal microvilli.
Presence of estrogen receptors
The presence of estrogenic receptors (Figure 3 and Table 4), was evidenced by the numerical increase in the mean of the pixels, in the connective tissue in the epididymal structures of the TCOU group of 21.5±0.9 compared to the C group which presented 6±0.5 (p>0.05).

Figure 3 Fluorescence in epididymis showing the presence of estrogen receptors (coumestrol). A. Scanty fluorescence in the epididymis of dogs of the control group, in which the decrease of epithelium stands out. B. Intense fluorescence in the epididymis of dogs treated with coumestrol in which there is evidence of increased connective tissue
Table 4 Fluorescence intensity associated with the presence of estrogen receptors
| Group/Quartile | Control Median±SE | Q1-Q3 Median | Coumestrol Median±SE | Q1-Q3 Median | Mann Whitney P |
|---|---|---|---|---|---|
| Epithelium | 1.4±0.1 | 0-3 | 3.1±0.4 | 0-5.7 | <0.01 |
| Connective tissue | 6.0±0.5 | 3-8 | 20.9±0.9 | 14.2-26 | >0.05 |
Fluorescence intensity/Green Pixels
Hormonal parameters
The hormone levels determined (Figure 4) showed differences between group C and the TCOU group. In-group C, testosterone levels were constant; on the other hand, there was a variation in estradiol levels. In the TCOU group, a decrease in testosterone levels, associated with an increase in estradiol levels, was evident from week four until week five.
DISCUSSION
Normal canine ejaculates should have at least 80% morphologically normal and viable spermatozoa (Chłopik & Wysokińska, 2020). In this study, normal sperm morphology of 60% or lower was found from the third week of treatment, which is similar to that reported in dogs considered infertile (Barbosa de Souza et al., 2015). The decrease in sperm concentration and the increase in spermatozoa with cytoplasmic droplet are evidence of the inhibitory effect of coumestrol on gonadal activity.
The sperm motility reported in fertile dogs should be located above 70% (Johnston,1991), in the present study in the dogs of the TCOU group from week 3 was located at 60% or lower, a value similar to that reported in infertile dogs (Barbosa de Souza et al., 2015). In the present study, secondary alterations of sperm morphology were found, which are associated with their maturation in the epididymis or during the preparation of semen samples. Among the main alterations were strongly coiled tail from week 3 and distal cytoplasmic droplet from week 4 post-treatment. A point to consider when interpreting these results is that the average time of passage through the epididymis of spermatozoa in domestic dogs is 15 days (3 weeks) (Chłopik & Wysokińska, 2020), the same time interval at which alterations began to be detected in the spermiogram.
Because the structure of phytoestrogens is similar to that of endogenous estrogens and they have the ability to bind to ER, it is possible to observe physiological effects in tissues expressing the receptor (Rietjens et al., 2017; Beszterda & Frański, 2018). Once the COU molecule binds to any of the estrogen receptors (ERα and ERβ) it can emit a fluorescent signal (Serrano et al., 2008; Wang et al., 2014). In the present study, such signal was detected with greater intensity in the connective tissue of the epididymis of dogs in the TCOU group, confirming that COU is absorbed and probably binds to estrogenic receptors present in this tissue. Pérez-Rivero et al. (2014) previously reported it in adult hematophagous bat (Desmodus rotundus) testes and Serrano et al., (2008) in male dogs, in both cases when COU was administered orally.
With respect to the echogenicity of the testicular parenchyma, it is homogeneous throughout the periphery, an area with decreased echogenicity is observed in the mediastinal region, which corresponds to the location of the vas deferens and the testicular network. It is consistent with the changes found at histological level related to the dilation of the tubules of the epididymis, loss of microvilli and decreased sperm content in its lumen (Mantziaras, 2020; Lubinus et al., 2006).
The process of estrogen biosynthesis depends on the enzyme aromatase activity, a member of the cytochrome P450 superfamily (Hess & Cooke, 2018). Aromatase is responsible for converting testosterone and androstenedione to aromatic estrogens, 17β-estradiol and estrone, respectively (Hess & Cooke, 2018). It has been described that COU can bind to the enzyme aromatase, resulting in inhibition of estrogen biosynthesis, so, it is possible to consider that the increase in serum testosterone concentration is due to this effect (Wyse et al., 2021; Lephart, 2015).
Estrogens are found in high concentrations in epithelial cells lining efferent ducts, their main function being to reabsorb the luminal fluid and thereby increase the concentration of spermatozoa. The biological effect absence of estrogens induces the accumulation of liquid in the efferent ducts, which finally generates dilatation of the same and of the rete testis. Subsequently there is a decrease in the epithelial height, in the number and height of the microvilli in the epididymis and subsequently atrophy of the seminiferous epithelium, all these changes favor semen dilution (Hess & Cooke, 2018; Pérez-Rivero et al., 2009).
CONCLUSION
The studies performed showed that subcutaneous administration of coumestrol could be a non-invasive alternative to decrease the fertilizing capacity of dogs, since it induced alterations in gonadal activity and cellularity associated with a decrease in sperm production and sperm quality. However, studies are still required to evaluate its possible reversible effect and sperm fertilizing capacity.
Funding
The study was supported by the Metropolitan Autonomous University Xochimilco Unit (UAM), research project DCBS.445.12.
Acknowledgments
National Council of Science and Technology (CONACYT) for the A.J.R. Scholarship for Doctoral Studies in Science.
The authors thank the animal protection shelter Quality of Life Foundation A.C., for providing their facilities and support in the care of the dogs (agreement celebrated with UAM-X dated 27 June 2016).
To Laura Karen Camarillo Rodea for her help in animal handling during the clinical evaluation and collection of biological samples.
To Susana Rojas Maya(†) for her generous support and technical assistance during the hormonal evaluation.
Conflict of interest
The authors declare that they do not have any conflict of interest
Authors' contribution
A.J.R: Designed and conducted the study, collected the data and biological samples, performed the laboratory work, analyzed and interpreted the results, drafted and approved the final manuscript.
J.J.P.R: Conceived the original idea and designed the study, supervised the study, analyzed and interpreted the data, performed critical revision of the manuscript, and approved the final manuscript.
J.A.H.B: Performed critical revision of the manuscript and approved the final manuscript. A.A.S: Performed critical revision of the manuscript and approved the final manuscript. M.P.M: erformed critical revision of the manuscript and approved the final manuscript.
LITERATURA CITADA
ALDER SA, Purup S, Hansen-Møller J, Thuen E, Steinshamn H. 2015. Phytoestrogens and their metabolites in bulk-tank milk: effects of farm management and season. PLoS One.10(5): e0127187. https://doi.org/10.1371/journal.pone.0127187 [ Links ]
ALI HASSAN H, Domain G, Luvoni GC, Chaaya R, Van Soom A, Wydooghe E. 2021. Canine and Feline Epididymal Semen-A Plentiful Source of Gametes. Animals.11:2961. https://doi.org/10.3390/ani11102961 [ Links ]
ASA CS. Contraception in Dogs and Cats. 2018. Vet Clin North Am Small Anim Pract. 48(4):733-742. https://doi.org/10.1016/j.cvsm.2018.02.014 [ Links ]
AZIZ SJ, Zeman-Pocrnich CE. 2022. Tissue Processing. Methods Mol Biol. 2422:47-63. https://doi.org/10.1007/978-1-0716-1948-3_4 [ Links ]
BARBOSA DE SOUZA M, England GCW, Mota Filho ACAckermann CL, Vládia Soares Sousa CV, Guedelha de Carvalho G, Rodrigues Silva HV, Pinto JN, Spíndola Linhares JC, Oba E, Machado da Silva LD. 2015. Semen quality, testicular B-mode and Doppler ultrasound, and serum testosterone concentrations in dogs with established infertility. Theriogenology. 84: 805-810. https://doi.org/10.1016/j.theriogenology.2015.05.015 [ Links ]
BELSARE A, Vanak AT. 2020. Modelling the challenges of managing free-ranging dog populations. Sci Rep. 10(1):18874. https://doi.org/10.1038/s41598-020-75828-6 [ Links ]
BESZTERDA M, Frański R. 2018. Endocrine disruptor compounds in environment: As a danger for children health. Pediatric Endocrinology Diabetes and Metabolism. 24(2):88-95. https://doi.org/10.18544/PEDM-24.02.0107 [ Links ]
CERUNDOLO R, Michel KE, Reisner IR, Phillips L, Goldschmidt M, Court MH, Shrestha B, Hao Q, Refsal K, Oliver JW, Biourge V, Shofer FS. 2009. Evaluation of the effects of dietary soy phytoestrogens on canine health, steroidogenesis, thyroid function, behavior and skin and coat quality in a prospective controlled randomized trial. American Journal of Veterinary Research. 70(3): 353-360. https://doi.org/10.2460/ajvr.70.3.353 [ Links ]
CHŁOPIC A, Wysokińska A. 2020. Canine spermatozoa-What do we know about their morphology and physiology? An overview. Reproduction in Domestic Animals. 55:113- 126. https://doi.org/10.1111/rda.13596 [ Links ]
COOKE PS, Mesa AM, Sirohi VK, Levin ER. 2021. Role of nuclear and membrane estrogen signaling pathways in the male and female reproductive tract. Differentiation. 118:24-33. https://doi.org/10.1016/j.diff.2020.11.002 [ Links ]
CORNWAL GA. 2009. New insights into epididymal biology and function. Human Reproduction Update. 15(2):213-227. https://doi.org/10.1093/humupd/dmn055 [ Links ]
DOMÍNGEZ-LÓPEZ I, Yago-Aragón M, Salas-Huetos A, Tresserra-Rimbau A, Hurtado- Barroso S. 2020. Effects of Dietary Phytoestrogens on Hormones throughout a Human Lifespan: A Review. Nutrients.12: 2456. https://doi.org/10.3390/nu12082456 [ Links ]
EVANS MJ, Gibson A, Fielding H, Ohal P, Pandey P, Kumar A, Singh SK, Airikkala-Otter I, Abela-Ridder B, Gamble L, Handel I, Bronsvoort BMDC, Mellanby RJ, Mazeri S. 2022. Free-roaming dog population dynamics in Ranchi, India. Research in Veterinary Science. 143:115-123. https://doi.org/10.1016/j.rvsc.2021.12.022 [ Links ]
HAMMER Ø, Harper DAT, Ryan PD. 2001. Past: Paleontological Statistics Software Package for Education and Data Analysis. Paleontología Electrónica. 4:1 https://palaeo-electronica.org/2001_1/past/issue1_01.htm [ Links ]
HAMPSON K, Abela-Ridder B, Bharti O, Knopf L, Léchenne M, Mindekem R, Tarantola A, Zinsstag J, Trotter C. 2019. Modelling to inform prophylaxis regimens to prevent human rabies. Vaccine. 37(1(Suppl 1)): A166-A173. https://doi.org/10.1016/j.vaccine.2018.11.010 [ Links ]
HAMPSON K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, Costa P, Freuling CM, Hiby E, Knopf L, Leanes F, Meslin FX, Metlin A, Miranda ME, Müller T, Nel LH, Recuenco S, Rupprecht CE, Schumacher C, Taylor L, Vigilato MA, Zinsstag J, Dushoff J. 2015. Global Alliance for Rabies Control Partners for Rabies Prevention. Estimating the global burden of endemic canine rabies. PLoS Neglected Tropical Diseases. 9(4): e0003709. https://doi.org/10.1371/journal.pntd.0003709 [ Links ]
HESS RA, Cooke PS. 2018. Estrogen in the male: a historical perspective. Biology of Reproduction. 99(1):27-44. https://doi.org/10.1093/biolre/ioy043 [ Links ]
JOHNSTON SD. 1991. Performing a complete canine semen evaluation in a small animal hospital. Veterinary Clinics of North America Small Animal Practice. 21(3):545-51. https://doi.org/10.1016/s0195-5616(91)50060-7 [ Links ]
KAWAKAMI E, Hirano T, Hori T, Tsutsui T. 2004. Improvement in spermatogenic function after subcutaneous implantation of a capsule containing an aromatase inhibitor in four oligozoospermic dogs and one azoospermic dog with high plasma estradiol-17b concentrations. Theriogenology. 62: 165-178. https://doi.org/10.1016/j.theriogenology.2003.09.021 [ Links ]
KUROWICKA B, Dietrich GJ, Kotwica G. 2015. Effect of neonatal or adult heat acclimation on testicular and epididymal morphometry and sperm production in rats. Reproductive Biology. 15(1):1-8. https://doi.org/10.1016/j.repbio.2015.01.001 [ Links ]
LEOCI R, Aiudi G, Silvestre F, Lissner EA, Marino F, Lacalandra GM. 2015. Therapeutic Ultrasound as a Potential Male Dog Contraceptive: Determination of the Most Effective Application Protocol. Reproduction in Domestic Animals. 50(5):712-8. https://doi.org/10.1111/rda.12548 [ Links ]
LEPHART ED. 2015. Modulation of Aromatase by Phytoestrogens. Enzyme Research. 594656. https://doi.org/10.1155/2015/594656 [ Links ]
LUBINUS BADILLO FG, Buitrago Aguilar C. 2006. Lesiones testiculares benignas: hallazgos ecográficos. Med UNAB. 9 (2):120-127. https://revistas.unab.edu.co/index.php/medunab/article/view/153 [ Links ]
MANTZIARAS G. 2020. Imaging of the male reproductive tract: Not so easy as it looks like. Theriogenology. 150:490e497. https://doi.org/10.1016/j.theriogenology.2020.03.009 [ Links ]
MASSEI G, Miller LA. 2013. Nonsurgical fertility control for managing free-roaming dog populations: a review of products and criteria for field applications. Theriogenology. 80(8):829-838. https://doi.org/10.1016/j.theriogenology.2013.07.016 [ Links ]
MIRANDA-CASTRO SP, Lizarraga-Paulin E. 2012. Is Chitosan a New Panacea? Areas of Application. In: The Complex World of Polysaccharides. (Karunaratne DN Ed). Intech Publisher, Croatia. Pp. 1-44. https://doi.org/10.5772/51200 [ Links ]
MORALES BM, Rodrigues da Rosa Filho, JR, Agostini LD, Infantosi VC. 2021. Ageing changes testes and epididymis blood flow without altering biometry and echodensity in dogs. Animal Reproduction Science. 228:106745. https://doi.org/10.1016/j.anireprosci.2021.106745 [ Links ]
MOTA-ROJAS D, Calderón-Maldonado N, Lezama-García K, Sepiurka L, Maria Garcia RC. 2021. Abandonment of dogs in Latin America: Strategies and ideas. Veterinary World. 14(9):2371-2379. https://doi.org/10.14202/vetworld.2021.2371-2379. [ Links ]
MOSTROM M, Evans TJ. 2018. Chapter 60 “Phytoestrogens”, Editor(s): Ramesh C. Gupta, Veterinary Toxicology (Third Edition), Academic Press, Pages 817-833, ISBN 9780128114100. https://doi.org/10.1016/B978-0-12-811410-0.00060-X [ Links ]
NCRRRAR. National Centre For The Replacement, Refinement, and Reduction of Animal in Research. The 3 R. http://www.nc3rs.org.uk/the-3rs [ Links ]
NIH. National Institutes of Health. Image Processing and Analysis in Java (ImageJ). http://rsb.info.nih.gov/ij/ [ Links ]
NIE R, Zhou Q, Jassim E, Saunders PTK, Hess RA. 2002. Differential Expression of Estrogen Receptors a and b in the Reproductive Tracts of Adult Male Dogs and Cats. Biology of Reproduction. 66:1161-1168. https://doi.org/10.1095/biolreprod66.4.1161 [ Links ]
PEÑA-CORONA S, León P, Mendieta E, Villanueva M, Salame A, Vargas D, Mora G, Serrano H, Villa-Godoy A. 2019. Effect of a single application of coumestrol and/or dimethyl sulfoxide, on sex hormone levels and vaginal cytology of anestrus bitches. Veterinaria México OA, 6(1), 52-66. Epub 20 de febrero de 2020. https://doi.org/10.22201/fmvz.24486760e.2019.1.656 [ Links ]
PEREZ-RIVERO JJ, Pérez-Martínez M, Aguilar-Setién A. 2014. Histometric analysis of vampire bat (Desmodus rotundus) testicles treated with coumestrol by oral route. Journal of Applied Animal Research. 42:2:208-212. https://doi.org/10.1080/09712119.2013.827578 [ Links ]
PEREZ-RIVERO JJ, Martinez-Maya JJ, Pérez-Martinez M, Aguilar-Setien A, García- Suarez MD, Serrano H. 2009. Phytoestrogen treatment induces testis alterations in dogs. Potential use in population control. Veterinary Research Communications. 33:87-95. https://doi.org/10.1007/s11259-008-9077-3 [ Links ]
RIETJENS IMCM, Louisse J, Beekmann K. 2017. The potential health effects of dietary phytoestrogens. British Journal of Pharmacology. 174(11):1263-1280. https://doi.org/10.1111/bph.13622 [ Links ]
ROOT KUSTRITZ MV. 2018. Population Control in Small Animals. Veterinary Clinics of North America Small Animal Practice. 48(4):721-732. https://doi.org/10.1016/j.cvsm.2018.02.013 [ Links ]
RUBEL D, Carbajo A. 2019. Dogs in public spaces of Buenos Aires, Argentina: Exploring patterns of the abundance of dogs, the canine faecal contamination, the behaviour of people with dogs, and its relationships with demographic/economic variables. Preventive Veterinary Medicine. 170:104713. https://doi.org/10.1016/j.prevetmed.2019.104713 [ Links ]
SANDAM NP, Prakash D, Thimmareddy P. 2021. Immunocontraceptive potential of a GnRH receptor-based fusion recombinant protein. Journal, genetic engineering & biotechnology. 19(1):63. https://doi.org/10.1186/s43141-021-00164-9 [ Links ]
SERRANO H, Pérez-Rivero JJ, Martínez-Maya JJ, Aguilar-Setién A, Pérez-Martinez M, García-Suárez MD. 2008. Fluorescence and inmunohistological detection of estrogen receptors in dog testis and epidydimis after oral coumestrol administration. Neuroendocrinology Letters. 29(6):977-980. https://doi.org/10.1007/s11259-008-9077-3 [ Links ]
SMITH LM, Hartmann S, Munteanu AM, Dalla Villa P, Quinnell RJ, Collins LM. 2019. The Effectiveness of Dog Population Management: A Systematic Review. Animals (Basel). 9(12):1020. https://doi.org/10.3390/ani9121020 [ Links ]
WANG D, Xie J, Zhu X, Li J, Zhao D, Zhao M. 2014. A recombinant estrogen receptor fragment-based homogenous fluorescent assay for rapid detection of estrogens. Biosensors and Bioelectronics. 55:391-395. https://doi.org/10.1016/j.bios.2013.12.050 [ Links ]
WYSE JM, Latif S, Gurusinghe S, Berntsen ED, Weston LA, Stephen CP. 2021. Characterization of Phytoestrogens in Medicago sativa L. and Grazing Beef Cattle. Metabolites.11(8): 550. https://doi.org/10.3390/metabo11080550s [ Links ]
ZUVELA E, Matson P. 2020. Performance of four chambers to measure sperm concentration: results from an external quality assurance programme. Reproductive biomedicine online. 41(4):671-678. https://doi.org/10.1016/j.rbmo.2020.07.008 [ Links ]
Received: February 27, 2022; Accepted: May 23, 2022











 texto en
texto en