Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Abanico veterinario
versão On-line ISSN 2448-6132versão impressa ISSN 2007-428X
Abanico vet vol.12 Tepic Jan./Dez. 2022 Epub 23-Jun-2023
https://doi.org/10.21929/abavet2022.21
Original Article
Morphometric identification of the predominant species of Varroa (Parasitiformes: Varroidae) in bee colonies in Hopelchén, Campeche
1Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias-Mocochá, Mérida, México.
2Colegio de Postgraduados, Campus Campeche, Sihochac, Champotón, Campeche. México.
3Instituto Tecnológico Superior de Hopelchén. Hopelchén, Campeche México.
4Unidad Académica de Medicina Veterinaria y Zootecnia, Universidad Autónoma de Nayarit, Compostela, Nayarit, México.
5Tecnológico Nacional de México/Instituto Tecnológico Superior de Calkiní. México.
Worldwide, varroasis continues to be the main sanitary problem in beekeeping production systems, causing great economic losses; currently in the Yucatan Peninsula the presence of Varroa has been reported, however, it is unknown which of the 4 species parasitize Apis mellifera bees in Campeche State. The aim of this research was to identify morphometrically the predominant species of Varroa (Parasitiformes: Varroidae) in bee colonies in Hopelchén, Campeche; for this purpose, 61 hives were evaluated from 5 apiaries, 200 to 300 bees were collected from each one; for the morphometric analysis, 244 mites were placed in 50% lactic acid for 2 hours at 100ºC, and then the segments were measured in an ocular micrometer. The results indicated that 100% of the mites evaluated belonged to the Varroa destructor species, the mean K clusters indicated intraspecific differences (P< 0.05), observing 5 morphotypes of V. destructor, the variables that presented greater variability were width of the anal shield (P=0.001) and width of the genital shield (P=0.001). It is concluded that although 100% of the mites belonged to V. destructor, they showed intraspecific morphometric differences.
Keywords: haplotype; varroasis; infestation; Apis
A nivel mundial, la varroasis continúa siendo el principal problema sanitario en los sistemas de producción apícola, causando grandes pérdidas económicas; actualmente en la península de Yucatán se ha reportado la presencia de Varroa, sin embargo, se desconoce cuál de las 4 especies parasitan a las abejas Apis mellifera en el Estado de Campeche. El objetivo de esta investigación fue identificar morfométricamente la especie predominante de Varroa (Parasitiformes: Varroidae) en colonias de abejas en Hopelchén, Campeche; para ello de 5 apiarios se evaluaron 61 colmenas, de cada una se colectaron de 200 a 300 abejas; para el análisis morfométrico, 244 ácaros fueron colocados en ácido láctico al 50% durante 2 horas a 100ºC, y posteriormente se midieron los segmentos en un micrómetro ocular. Los resultados indicaron que el 100% de los ácaros evaluados pertenecen a la especie de Varroa destructor, los conglomerados K medias indicaron diferencias intraespecíficas (P< 0.05) observándose 5 morfotipos de V. destructor, las variables que presentaron mayor variabilidad fueron ancho del escudo anal (P=0.001) y ancho del escudo genital (P=0.001). Se concluye que a pesar de que el 100% de los ácaros pertenecieron a V. destructor estos presentaron diferencias morfométricas intraespecíficas.
Palabras clave: haplotipo; varroasis; infestación; Apis
INTRODUCTION
Varroasis is a disease caused by the Varroa mite, an obligate ectoparasite of honey bees (Rosenkranz et al., 2010). To date, four species of the genus Varroa are known, including Varroa jacobsoni and Varroa underwoodi, mites that parasitize Apis cerana bees and they are distributed throughout Asia, Varroa rindereri described in Apis koschevnikovi bees and distributed in Borneo, and Varroa destructor described in both Apis cerana and Apis mellifera bees. The presence of V. destructor was first recorded in the Americas in 1987 and recently through morphometric studies it has been identified in countries such as Mexico and Argentina (De Guzman & Delfinado, 1996; De Guzman & Rinderer, 1999; Anderson & Trueman, 2000; Anderson, 2000a; Maggi et al., 2009; Loeza-Concha et al., 2018).
Despite the existence of four species of the genus Varroa only V. destructor is considered of economic importance, since untreated colonies can collapse due to the presence of this mite after 3 to 4 years of the initial infestation (BüChler, 1994). Mites are harmful because they generally lodge in the thorax and abdomen of drones and worker bees, besides feeding mainly on fat bodies of adult bees and the hemolymph of larvae (Ramsey et al., 2019), which causes serious damage to the health of bees affecting the immune system, reduces the growth and development of colonies (Moreira et al., 2017). In this sense, it has been observed that worker bees have a reduced lifespan, have a lower learning ability and lower rate of return to the colony (Amdam et al., 2004; Kralj et al., 2007). In addition, V. destructor is considered a vector of several honeybee viruses (Chen & Siede, 2007) because viruses have been considered a problem for honeybee health since their emergence (Yue & Genersch, 2005).
Recent studies have shown that bee size correlates with mite size, whereby V. jacobsoni affects Apis cerana, being smaller than V. destructor and A. mellifera (Anderson & Trueman, 2000), to test this hypothesis morphometric discrimination techniques have been used by measuring body segments, which mainly use concepts of size and shape in order to know the morphological adaptations (Delfinado & Houck, 1989). In this sense authors such as De Guzman & Delfinado-Baker (1996), Anderson & Trueman (2000), Maggi et al., (2009), Loeza-Concha et al., (2018) have studied the morphometric variations of different populations of Varroa mites where different morphotypes have been established in different regions of the world. In this region until now it was unknown which of the four species affects the different populations of A. mellifera and if there are morphological variations in Varroa populations that affect the different populations of A. mellifera. The objective of this research was to identify morphometrically the predominant species of Varroa (Parasitiformes: Varroidae) in bee colonies in Hopelchén, Campeche.
MATERIAL AND METHODS
Experimental area location
The research was carried out in the apiaries of the Higher Technological Institute of Hopelchén, Campeche, located at 19°76′41″ north latitude and 89°86′68″ west longitude at 100 m a.s.l. Two types of climates predominate in the area: warm sub-humid (awo) (w), with summer rainfall of less than 5.0 mm and warm sub-humid (aw1), with winter rainfall and precipitation between 5 and 10.2 mm. The average annual precipitation is 1,050 mm, with rainfall from May to October. The annual temperature varies between 19.5 °C and 32.5 °C, with an average of 26 °C (Weather Spark, 2021).
Sample obtaining
The research was carried out in 5 apiaries in Hopelchén municipality where 61 hives were studied with the following characteristics: five brood frames in all stages of development and 4 frames with honey and pollen. In these hives, 200 to 300 bees were collected from the third and fourth frames of the brood chamber. Bees were placed in containers with absolute alcohol until use (Loeza-Concha et al., 2018).
Varroa specimen collection
Varroa specimens were obtained using the methodology described by De Jong et al., (1982) with modifications (Loeza-Concha et al., 2020), which consisted of shaking plastic containers containing between 200 and 300 bees for 10 min at 60 rpm. The contents of containers were placed in a conical container with a 3 mm mesh which was filled with absolute alcohol until the bees were completely covered, then, with a glass rod the samples were shaken to detach mites from bees, so that by gravity the mites were deposited at the bottom of the cone, finally, the solution was decanted through a white cloth and mites obtained from each of the hives were stored and labeled separately in microtubes of 1. 5 ml microtubes and kept refrigerated (4 °C) until use.
Mite processing
To determine the predominant Varroa species and morphometric variability, 244 female Varroa specimens were analyzed and placed in 50% lactic acid for 2 h at 100 °C; subsequently, mites were stored in 50% v/v alcohol until observation. Morphometric characters were measured using a stereo microscope with an ocular micrometer at 20X (Maggi et al., 2009; Loeza-Concha et al., 2018).
Morphometry
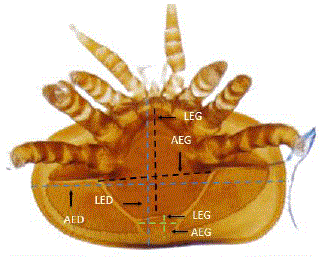
For morphometric identification of the predominant Varroa specimens, six variables were measured on each specimen: dorsal shield width (AED), dorsal shield length (LED), genital shield width (AEG), genital shield length (LEG), anal shield width (AEA) and anal shield length (LEA) (Figure 1) (Maggi et al., 2009; Loeza-Concha et al., 2018).
Statistical analysis
To determine the morphometric differences of Varroa between apiaries, a comparison of means was performed with a one-factor ANOVA test; the variables that had significant differences were subjected to a second post hoc multiple comparison analysis using a Tukey's comparison of means (P<0.001). To determine the morphotypes, a K-means cluster analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 20.0 (IBM, 2011).
RESULTS
It was determined that 100% of the specimens evaluated in this study area belonged to the species V. destructor, only the variables GSW and ASW showed differences among the apiaries evaluated (Table 1).
Table 1 Mean of the studied variables (µm) belonging to the V. destructor populations of five evaluated apiaries
| Apiarie | DSW | DSL | GSW | GSL | ASW | ASL |
| 1 | 1685a | 1159a | 688a | 733a | 248abc | 199a |
| 2 | 1688a | 1153a | 707ab | 734a | 261c | 198a |
| 3 | 1691a | 1151a | 719b | 761a | 238ab | 197a |
| 4 | 1689a | 1154a | 711ab | 741a | 254bc | 206a |
| 5 | 1684a | 1146a | 708ab | 755a | 233a | 201a |
| Mean | 1687 | 1553 | 703 | 742 | 248 | 200 |
| SEM | 3.36 | 2.38 | 2.89 | 3.77 | 2.36 | 1.87 |
Dorsal shield width (DSW), dorsal shield length (DSL), genital shield width (GSW), genital shield length (GSL), anal shield width (ASW) and anal shield length (ASL). Standard error of the mean (SEM). Different literals per column indicate Tukey statistical difference with p<0.001
According to the analysis of hierarchical clusters K means, it could be observed that all the variables presented discrimination for the formation of clusters among the Varroa populations analyzed in the five apiaries. In this sense, 5 morphotypes of V. destructor, which allowed us to observe that morphotypes A and E were the most widely distributed; these were differentiated because morphotype A presented smaller DSW size and larger DSL size compared to morphotype E. It was observed that morphotype B was the least distributed, presenting a larger DSW and smaller DSL compared to morphotypes A and E (Table 2).
Table 2 Mean of the studied variables (µm) belonging to the 5 morphotypes of V. destructor
| Morphotype | DSW | DSL | DSW | GSL | ASW | ASL |
| A | 1681 | 1165 | 675 | 754 | 239 | 184 |
| B | 1706 | 1144 | 712 | 837 | 237 | 215 |
| C | 1652 | 1149 | 709 | 736 | 230 | 205 |
| D | 1684 | 1144 | 693 | 712 | 256 | 197 |
| E | 1707 | 1159 | 717 | 752 | 255 | 204 |
| p< | 0.0001 | 0.02 | 0.0001 | 0.0001 | 0.0001 | 0.002 |
Dorsal shield width (DSW), dorsal shield length (DSL), genital shield width (GSW), genital shield length (GSL), anal shield width (ASW) and anal shield length (ASL)
According to the cluster analysis, it was observed that there is a morphometric variability of the mites in the 5 apiaries evaluated. In this sense, of the 5 morphotypes found, morphotypes A and E were the most widely distributed since they were found in hives of the 5 apiaries. Morphotype B was the least distributed since it was only found in hives of apiaries 1 and 5; morphotype C in hives of apiaries 1, 3, 4 and 5, morphotype A in hives of apiaries 1, 2, 4 and 5 (Table 3).
Table 3 Number of colonies belonging to each morphotype per apiary
| Morphotype | A | B | C | D | E |
| Apiary | Number of hives colmenas /Apiary | ||||
| 1 | 4 | 1 | 4 | 7 | 3 |
| 2 | 2 | -- | -- | 5 | 5 |
| 3 | 1 | -- | 2 | -- | 4 |
| 4 | 1 | -- | 2 | 2 | 8 |
| 5 | 1 | 1 | 3 | 1 | 4 |
| Total | 9 | 2 | 11 | 15 | 24 |
According to the data obtained, when plotting the similarities between the morphotypes, a grouping in three nodes is observed, isolating morphotype B with higher values of LEG and LEA, but lower number of colonies (Figure 2, Table 2).
DISCUSSION
The results obtained indicate that the predominant species of Varroa in the 5 apiaries analyzed is V. destructor since according to Abou-Shaara & Tabikha, (2016). The proportion of body size is equal or greater than 1140 µm, confirming that the existing species is V. destructor. Our results agree with those obtained in Argentina by Maggi et al. (2009) where they reported 3 mite morphotypes, with a range of DSW 1696 µm to 1757 µm and DSL from 1128 µm to 1178 µm. For the case of Mexico, Loeza-Concha et al. (2018) found 8 morphotypes of V. destructor species with DSW ranges from 1582 µm to 1700 µm and DSL from 1042 µm to 1147 µm. In Japan, Thailand and Vietnam, Anderson & Trueman, (2000) reported the presence of V. destructor with a DSW of 1708 µm and a DSL of 1167 µm. In New Zealand, Zhang, (2000) reported that the DSL ranges of V. destructor were from 1132 µm to 1185 µm and the DSW 1642 µm to 1757 µm. In Poland where they studied the cell size effect so it could be demonstrated that the mite of V. destructor significantly reduced its size when the DSW of V. destructor was reduced when the V. destructor DSW was 1132 µm to 1757 µm. V. destructor mite significantly reduced its size when it was housed in small cell sizes. In this sense, Borsuk et al. (2012) reported DSW and DSL of 1665 µm and 1121 µm respectively in small cell sizes and 1716 µm and 1142 µm respectively in standard cell sizes. We can mention several similar reports in Benin, Nigeria, Tunisia, Iran and Egypt (Table 4) (Rahmani et al., 2006; Akinwande et al., 2013; Abou-Shaara & Tabikha, 2016; Kelomey et al., 2016; Yevstafieva & Nasarenko, 2018), according to the above mentioned, V. destructor is distributed in most of the world. This mite presents variations in size and shape within the same mite population in the different bee species it parasitizes (Akimov et al., 2004). In this sense, we consider that the morphometric variability observed in this mite can be defined as an adaptation adjustment to the environment, which allows maintaining the individual fitness of the mite and the subsistence of the species (Pigliucci, 2005; Nussey et al., 2007).

Figura 2 Grouping of morphotypes by similarity considering morphometric variables (morphotypes A, B, C, D and E)
According to the above, we consider that the variations of Varroa morphotypes found in the study area are mainly due to the interaction between the mite and bee species it parasitizes. Thus, we agree with Giménez et al. (2017) and George et al. (2004), who indicate that parasites tend to vary their morphotype according to their host, i.e. morphometric variability depends on the lineage of Apis mellifera that the mite parasitizes. The results obtained with the analysis of hierarchical clusters K means (Table 2) it could be observed that there is morphometric variability between closely related populations (Figure 1). Loeza-Concha et al. (2018) found 8 morphotypes in Tepic, Nayarit, Mexico, likewise, Maggi et al. (2009) found 7 morphotypes of V. destructor in colonies located in different geographical areas of Argentina, similarly, Akimov et al. (2004) and Dadgostar & Nozari (2018) have reported morphometric and gyiographic differences of Varroa mites in Iran and Ukraine. According to the aforementioned we differ with Rosenkranz et al. (2010) quienes who indicated that Varroa mites from different populations are physically the same; as well as with Dadgostar & Nozari (2018) indicated that geographical variations are causes of Varroa morphological variations. In this sense, we consider that if there are physical and morphometric differences between different Varroa populations since it not only depends on geographical variation but also on colony migration, morphometric correlations between coexisting V. destructor populations and bee species it parasitizes and the mutations that the mite may present (Akimov et al., 2004; Loeza- Concha et al., 2018). These morphometric differences in arthropods and insects has been reported previously (Mozaffarian et al., 2007; Lashkari et al., 2015).
Table 4 Body size measurements in micrometers of Varroa females in the world
| Taxonomic group (species) | DSL (µm) | DSW (µm) | Origin | Author |
|---|---|---|---|---|
| V. rindereri | 1180 | 1698 | Malaysia | De Guzman & Delfinado (1996) |
| V. destructor | 1167 | 1708 | Japan /Thailand/ Vietnam | Anderson & Trueman (2000) |
| V. Jacobsoni | 1063 | 1506 | Java | Anderson (2000b) |
| V. destructor | 1159 | 1700 | ||
| V. destructor | 1167 | 1708 | New Zealand | Zhang (2000) |
| V. Jacobsoni | 1063 | 1506 | ||
| V. destructor | 1205 | 1738 | North Tunisia | |
| V. destructor | 1165 | 1711 | Central Tunisia | Boudagga et al. (2003) |
| V. destructor | 1197 | 1756 | South Tunisia | |
| V. destructor | 1149 | 1692 | Ukraine | Akimov et al. (2004) |
| V. destructor | 1197 | 1775 | Iran Colonies less than 1000 m of altitude | |
| V. destructor | 1199 | 1781 | Iran Colonies between 1000- 1500 m altitude | Rahmani, et al. (2006) |
| V. destructor | 1200 | 1789 | Iran Colonies at more than 1500 m altitude | |
| V. destructor | 1135 | 1696 | ||
| V. destructor | 1128 | 1711 | Argentina | Maggi et al. (2009) |
| V. destructor | 1178 | 1757 | ||
| V. destructor | 1121 | 1665 | ||
| Poland | Borsuk et al. (2012) | |||
| V. destructor | 1142 | 1716 | ||
| V. destructor | 1177 | 1718 | Nigeria | Akinwande et al. (2013) |
| V. destructor | 1115 | 1639 | Benin | Kelomey et al. (2016) |
| V. destructor | 1160 | 1710 | Egypt | Abou-Shaara & Tabikha (2016) |
| V. destructor | 1128 | 1688 | Nayarit, Mexico | Loeza-Concha et al. (2018) |
| V. destructor | 1090 | 1630 | Ukraine | Yevstafieva & Nasarenko (2018) |
Width of dorsal shield (DSW); length of dorsal shield (DSL)
Finally and according to the morphometric data obtained in this research we agree with Akimov et al. (2004) y Abou-Shaara & Tabikha (2016), since we consider that according to the body characteristics of V. destructor specimens obtained in Mexico. It is suggested that they are of the Korean haplotype, especially because the mean values of the length and width of the dorsal shield are similar to those found in this research (1149 µm and 1692 µm). Besides that the Korean haplotype is the most common worldwide since records of its presence are found in Europe, Middle East, Africa, Asia, North America and South America (Zhang, 2000; Muñoz et al., 2008; Akinwande et al., 2012). The present investigation takes on greater relevance if we consider that the Japanese Varroa haplotype has a more restricted distribution. It is considered less virulent compared to the Korean haplotype which reproduces more easily (De Guzman & Rinderer 1999), because of this Carneiro et al. (2007) indicate that the reproduction rate of V. destructor females in young worker cells of Africanized honeybees in Brazil is currently almost double compared to the reproduction rate of twenty years ago. It is considered that Varroa populations of the Japanese haplotype have been replaced by the Korean haplotype, causing an increase in infestation levels in South America (Strapazzon et al., 2009), which could lead to a greater loss of hives in America.
LITERATURA CITADA
ABOU-SHAARA H, Tabikha R. 2016. Morphological characterization and a morphometry map for Varroa mites from northwest of Egypt. Cercetari Agronomice in Moldova. 49(4):75-84. ISSN: 2067-1865. https://doi.org/10.1515/cerce-2016-0038 [ Links ]
AKIMOV I, Benedyk S, Zaloznaya L. 2004. Complex analysis of morphological characters of Gamasid mite Varroa destructor (Parasitiformes, Varroidae). Vestnik Zoologii. 38(5): 57-66. ISSN: 0084-5604, 20732333. http://dspace.nbuv.gov.ua/handle/123456789/3366 [ Links ]
AKINWANDE K, Badejo M, Ogbogu S. 2013. Morphometrics and parasitic load of Varroa mites (Acari: Varroidae) on colonies of Apis mellifera adansonii (Hymenoptera: Apidae) in South Western, Nigeria. Acarina. 21(1):17-26. ISSN: 0132-8077, 22215115 https://acarina.utmn.ru/upload/iblock/b39/Akinwande2013.pdf [ Links ]
AKINWANDE KL, Badejo MA, Ogbogu SS. 2012. Incidence of the Korean haplotype of Varroa destructor in southwest Nigeria. Journal of apicultural research. 51(4):369-370. ISSN: 0021-8839. https://doi.org/10.3896/IBRA.1.51.4.15 [ Links ]
AMDAM GV, Hartfelder K, Norberg K, Hagen A, Omholt SW. 2004. Altered physiology in worker honey bees (Hymenoptera: Apidae) infested with the mite Varroa destructor (Acari: Varroidae): a factor in colony loss during overwintering?. Journal of economic entomology. 97(3):741-747. ISSN 1938-291X. https://doi.org/10.1093/jee/97.3.741 [ Links ]
ANDERSON D, Trueman J. 2000a. Varroa jacobsoni (Acari: Varroidae) is more than one species. Experimental and applied acarology. 24(3):165-189. ISSN: 1572-9702. https://link.springer.com/content/pdf/10.1023/A:1006456720416.pdf [ Links ]
ANDERSON D. 2000b. Variation in the parasitic bee mite Varroa jacobsoni Oud. Apidologie. 31(2):281-292. ISSN: 0044-8435. https://agris.fao.org/agris-search/search.do?recordID=FR2000003100 [ Links ]
BORSUK G, Olszewski K, Strachecka A, Paleolog J, Kasperek K. 2012. Genetic and morphometric variation of the Varroa destructor developing in standard and small comb cells. Veterinary Medical Science and Practice (Medycyna Weterynaryjna). 68:599-602. http://www.medycynawet.edu.pl/images/stories/pdf/pdf2012/102012/201210599602.pdf [ Links ]
BOUDAGGA H, Barbouche N, Laârif A, Hamouda MH. 2003. Morphological identification of the Varroa species (Acari: Varroidae) colonizing Tunisian apiaries. Systematic and Applied Acarology. 8(1):97-100. ISSN: 1362-1971. https://doi.org/10.11158/saa.8.1.12v [ Links ]
BÜCHLER R. 1994. Varroa tolerance in honey bees-occurrence, characters and breeding. Bee World Journal. 75(2):54-70. ISSN: 0005-772X. https://doi.org/10.1080/0005772X.1994.11099201 [ Links ]
CARNEIRO FE, Torres RR, Strapazzon R, Ramírez SA, Guerra Jr, JC, Koling DF, Moretto G. 2007. Alteração do potencial reprodutivo do ácaro Varroa destructor (Anderson e Trueman) em colônias de abelhas africanizadas (Apis mellifera L.) (Hymenoptera: Apidae) no sul do Brasil. Entomología Neotropical. 36(6):949-952. ISSN: 1678-8052. https://www.scielo.br/j/ne/a/hZQsr7tqBsCs75DV4sWKzFQ/abstract/?lang=en [ Links ]
CHEN Y, Siede R. 2007. Honey bee viruses. Advances in virus research. 70:33-80. ISSN: 0065-3527, 1557839. https://doi.org/10.1016/S0065-3527(07)70002-7 [ Links ]
DE GUZMAN L, Delfinado-Baker M. 1996. A new species of Varroa (Acari: Varroidae) associated with Apis koschevnikovi (Apidae: Hymenoptera) in Borneo. International Journal of Acarology. 22(1):23-27. ISSN: 1647954. https://doi.org/10.1080/01647959608684077 [ Links ]
DE GUZMAN L, Rinderer T. 1999. Identification and comparison of Varroa species infesting honey bees. Apidologie. 30(2-3):85-95. ISSN: 0044-8435. https://doi.org/10.1051/apido:19990201 [ Links ]
DE JONG D, De Jong P, Goncalves L. 1982. Weight Loss and Other Damage to Developing Worker Honeybees from Infestation With Varroa jacobsoni. Journal of apicultural research. 21(3):165-167. ISSN: 0021-8839. https://doi.org/10.1080/00218839.1982.11100535 [ Links ]
DELFINADO M, Houck M. 1989. Geographic variation in Varroa jacobsoni (Acari, Varroidae): application of multivariate morphometric techniques. Apidologie, 20(4):345-358. ISSN: 0044-8435. https://www.apidologie.org/articles/apido/pdf/1989/04/Apidologie_0044- 8435_1989_20_4_ART0007.pdf [ Links ]
DADGOSTAR S, Nozari J. 2018. Classical and geometric morphometric methods reveal differences between specimens of Varroa destructor (Mesostigmata: Varroidae) from seven provinces of Iran. Persian journal of acarology. 7(1):51-60. ISSN: 2251-8169. https://doi.org/10.22073/pja.v7i1.32063 [ Links ]
GIMÉNEZ P, Mendoza Y, Invenizzi C, Fuselli S, Alonso R, Fernandez P, Maggi M. 2017. Morphometric correlation between Apis mellifera morphotypes (Hymenoptera) and Varroa destructor (Acari) from Uruguay. Journal of Apicultural Research. 56(2): 122-129. ISSN: 0021-8839. https://doi.org/10.1080/00218839.2017.1287998 [ Links ]
GEORGE M, Muñoz G, Marquet P, Poulin R. 2004. Testing the energetic equivalence rule with helminth endoparasites of vertebrates. Ecology Letters. 7(7): 527-531. ISSN: 1461-0248. https://doi.org/10.1111/j.1461-0248.2004.00609.x [ Links ]
KELOMEY E, Paraiso A, Azonwade F, Gbemavo C, Goergen, GE, Tamo M, Baba-Moussa L. 2016. Morphometric characterization of parasite Varroa sp. of bee Apis mellifera L. in Benin. European Scientific Journal. 12(33):221-234. ISSN: 1857-7881. http://dx.doi.org/10.19044/esj.2016.v12n33p221 [ Links ]
KRALJ J, Brockmann A, Fuchs S, Tautz J. 2007. The parasitic mite Varroa destructor affects non-associative learning in honey bee foragers, Apis mellifera L. Journal of Comparative Physiology A. 193(3):363-370. ISSN: 1432-1351. http://dx.doi.org/10.1007/s00359-006-0192-8 [ Links ]
IBM SPSS. 2011. IBM SPSS statistics for Windows, version 20.0 (p. 440). New York: IBM Corp. [ Links ]
LASHKARI M, Hentz MG, Boykin LM. 2015. Morphometric comparisons of Diaphorina citri (Hemiptera: Liviidae) populations from Iran, USA and Pakistan. Peer J. 3, e946. 1-12. ISSN: 2689-7733. https://doi.org/10.7717/peerj.946 [ Links ]
LOEZA-Concha H, Salgado-Moreno S, Avila-Ramos F, Escalera-Valente F, Lemus- Flores C, Domínguez-Rebolledo Á, Carmona-Gasca C. (2020). Seasonal variation in the prevalence of Varroa, Nosema and Acarapis in hives from which queen bee mating nuclei are produced. Journal of Apicultural Research. 59(4): 558-563. ISSN: 0021-8839. https://doi.org/10.1080/00218839.2020.1717060 [ Links ]
LOEZA-Concha H, Domínguez-Rebolledo A, Escalera-Valente F, Ávila-Ramos F, Carmona-Gasca C. 2018. Identificación morfométrica de Varroa destructor y su plasticidad por la exposición a timol. Abanico veterinario. 8(2):98-107. ISSN 2448-6132. https://doi.org/10.21929/abavet2018.82.9 [ Links ]
MAGGI M, Sardella N, Ruffinengo S, Eguaras M. 2009. Morphotypes of Varroa destructor collected in Apis mellifera colonies from different geographic locations of Argentina. Parasitology research, 105(6):1629-1636. ISSN: 1432-1955. https://doi.org/10.1007/s00436-009-1605-8 [ Links ]
MOREIRA S, Queiroz G, De Castro H, De Souza E, Pereira D, De Holanda J. 2017. Infestação do ácaro Varroa destructor em colônias de abelhas africanizadas (Apis mellifera L.) no Semiárido potiguar, Nordeste do Brasil. Revista Verde de Agroecologia e Desenvolvimento Sustentável. 12(1):143-149. ISSN 1981-8203. http://dx.doi.org/10.18378/rvads.v12i1.4845 [ Links ]
MOZAFFARIAN F, Sarafrazi A, Ganbalani GN. 2007. Host plant-associated population variation in the carob moth Ectomyeloisceratoniae in Iran: A geometric morphometric analysis suggests a nutritional basis. Journal of Insect Science. 7(1). ISSN 1536-2442. https://doi.org/10.1673/031.007.0201 [ Links ]
MUÑOZ I, Garrido-Bailón E, Martín-Hernández R, Meana A, Higes M, De la Rúa P. 2008. Genetic profile of Varroa destructor infesting Apis mellifera iberiensis colonies. Journal of apicultural research. 47(4):310-313. ISSN: 0021-8839. https://doi.org/10.1080/00218839.2008.11101480 [ Links ]
NUSSEY D, Wilson A, Brommer J. 2007. The evolutionary ecology of individual phenotypic plasticity in wild populations. Journal of evolutionary biology. 20:831-844. ISSN: 1420-9101. https://doi.org/10.1111/j.1420-9101.2007.01300.x [ Links ]
PIGLIUCCI M. 2005. Evolution of phenotypic plasticity: where are we going now? Trends in Ecology y Evolution. 20:481-486. ISSN: 0169-5347. https://doi.org/10.1016/j.tree.2005.06.001 [ Links ]
RAHMANI H, Sabouri A, Nozari J, Kamali K. 2006. Report and survey of morphometric characteristics of Varroa destructor (acari: varroidae) collected from honey bees in Tehran province, Iran (Research note). Journal of Agricultural Science and Technology. 8(1):351-355.ISSN: 1680-7073. https://www.sid.ir/en/journal/ViewPaper.aspx?id=47578 [ Links ]
RAMSEY S. Ochoa R. Bauchan G, Gulbronson C, Mowery J, Cohen A, Ellis J. 2019. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proceedings of the National Academy of Sciences. 116(5):1792-1801. ISSN: 0027-8424. https://doi.org/10.1073/pnas.1818371116 [ Links ]
ROSENKRANZ P, Aumeier P, Ziegelmann B. 2010. Biology and control of Varroa destructor. Journal of invertebrate pathology. 103:S96-S119. ISSN: 0022-2011, 10960805. https://doi.org/10.1016/j.jip.2009.07.016 [ Links ]
STRAPAZZON R, Carneiro F, Guerra Jr J, Moretto G . 2009. Genetic characterization of the mite Varroa destructor (Acari: Varroidae) collected from honey bees Apis mellifera (Hymenoptera, Apidae) in the state of Santa Catarina. Brazil. Genetics and Molecular Research. 8(3):990-997. ISSN: 0022-2011, 10960805. https://www.geneticsmr.org/articles/genetic-characterization-of-the-mite-varroa-destructor-acari-varroidae-collected-from-honey-bees-apis-mellifera-hymenopt.pdf [ Links ]
WEATHER Spark 2021. El clima promedio en Hopelchén, Campeche, México. https://es.weatherspark.com/y/12357/Clima-promedio-en-Hopelchen-M%C3%A9xico-durante-todo-el-a%C3%B1o [ Links ]
YEVSTAFIEVA V, Nasarenko O. 2018. Морфометричні ознаки самок Varroa destructor Anderson and Trueman, 2000 (Acari, Mesostigmata: Varroidae). Theoretical and Applied Veterinary Medicine. 6(1):40-45. ISSN: 2090-3308. https://bulletin-biosafety.com/index.php/journal/article/view/169 [ Links ]
YUE C, Genersch E. 2005. RT-PCR analysis of Deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). Journal of General Virology. 6(12):3419-3424. ISSN: 0022-1317, 14652099. https://doi.org/10.1099/vir.0.81401-0 [ Links ]
ZHANG Z. 2000. Notes on Varroa destructor (Acari: Varroidae) parasitic on honeybees in New Zealand. Systematic and Applied Acarology Special Publications. 5(1):9-14. ISSN: 1362-1971. https://doi.org/10.11158/saasp.5.1.2 [ Links ]
Received: January 18, 2022; Accepted: July 27, 2022











 texto em
texto em