Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Abanico veterinario
versión On-line ISSN 2448-6132versión impresa ISSN 2007-428X
Abanico vet vol.11 Tepic ene./dic. 2021 Epub 21-Mayo-2021
https://doi.org/10.21929/abavet2021.10
Short Communication
Preliminary study on the in vitro inhibition of gastrointestinal nematodes from sheep with aqueous extracts of forage plants
1Colegio de Postgraduados, Campus Campeche. Carretera Haltunchén-Edzná, km. 17.5, Champotón, Campeche, México. CP. 24050.
2CONACYT-Colegio de Postgraduados, Campus Campeche. Carretera Haltunchén-Edzná, km. 17.5, Champotón, Campeche, México. CP. 24050.
3Departamento de Veterinaria y Zootecnia. Campus Irapuato-Salamanca, Universidad de Guanajuato, km 9. Carretera Irapuato-Silao, Exhacienda El Copal, Irapuato, Guanajuato. CP. 36824.
The presence of gastrointestinal nematodes (GIN) in sheep is a low productivity cause. This study aimed to determine the in vitro efficiency of Gliricidia sepium, Leucaena leucocephala, Guazuma ulmifolia, and Bursera simaruba aqueous extracts at three different doses (0.75, 1.00, and 1.25 mL), inhibiting the egg hatching of GIN from sheep. The inhibition efficiency in egg hatching (IEH), larval identification, and its genera proportion were evaluated. Analysis of data was performed using Chi-square tests and analysis of variance. The four extracts obtained an IEH of 50%, being similar (p > 0.05) between them. The 1.25 mL dose and its combinations of Leucaena leucocephala and Gliricidia sepium at 1.25 mL dose obtained the highest efficiency (68.0, 85.0, and 77.0%, respectively). Five genera of larva were identified (Haemonchus spp, Trichostrongylus spp, Oesophagostomum spp, Cooperia spp, and Nematodirus spp). The highest prevalence (p ≤ 0.05) was obtained by Haemonchus spp (58.0%). According to the results, the four aqueous extracts exhibited ovicidal activity (GIN). However, the 1.25 mL dose and the Leucaena leucocephala, Gliricidia sepium extracts at 1.25 mL doses were the most effective.
Keywords: Gastrointestinal parasites; anthelmintic plants; egg hatching
La alta presencia de nematodos gastrointestinales (ngi) en ovinos, es una causa de baja productividad. El objetivo fue determinar de forma preliminar la eficiencia in vitro de extractos acuosos de Gliricidia sepium, Leucaena leucocephala, Guazuma ulmifolia y Bursera simaruba a tres dosis (0.75, 1.00 y 1.25 mL), en la inhibición de la eclosión de huevos de ngi de ovinos. Se evaluó la eficiencia de inhibición en la eclosión de huevos (EIH), identificación y proporción de géneros de larvas. El análisis de datos se realizó mediante pruebas de Chi cuadrada y análisis de varianza. Los cuatro extractos obtuvieron una EIH del 50%, siendo similares (p > 0.05) entre ellos. La dosis de 1.25 mL y las combinaciones de Leucaena leucocephala y Gliricidia sepium a dosis de 1.25 mL obtuvieron mayor eficiencia (68.0, 85.0 y 77.0%, respectivamente). Se identificaron cinco géneros de larvas (Haemonchus spp., Trichostrongylus spp., Oesophagostomum spp., Cooperia spp., y Nematodirus spp.), siendo el de mayor (p ≤ 0.05) prevalencia Haemonchus spp. (58.0%). Los cuatro extractos acuosos exhibieron actividad ovicida de ngi. No obstante, la dosis de 1.25 mL y los extractos de Leucaena leucocephala, Gliricidia sepium a dosis de 1.25 mL fueron los más eficaces.
Palabras clave: Parásitos gastrointestinales; plantas antihelmínticas; eclosión de huevos
INTRODUCTION
In Mexico, the economic impact derived from parasitism caused by gastrointestinal nematodes (GIN) is 445 million dollars per year (Rodríguez-Vivas et al., 2017), representing a serious problem by affecting animal productivity; translating into appetite loss, weight, anemia, diarrhea, growth retardation and even death (Rodríguez-Martínez et al., 2018). Chemical anthelmintics have been used for GIN control for decades because of their broad spectrum and ease of use. However, the irrational use of them (Closantel, Albendazole, Ivermectin and Nitroxinil), has developed resistance in GIN (Mondragón- Ancelmo et al., 2019) as has been reported for the genera Haemonchus contortus, Teladorsagia circumcincta, Tricostrongylus spp., and Nematodirus spp., (Holsback et al., 2016).
Currently, novel and sustainable alternatives are required for the control of GIN, such as the use of forage plants, which apart from offering benefits due to their nutritional quality, have anthelmintic action; which has been related to the presence of secondary metabolites, among which are: lectins, terpenes, alkaloids, saponins, anthraquinones, flavonoids and tannins (Oliveira et al., 2017); the latter being those that have been associated mainly in the vital functions of nematodes (Medina et al., 2014). These have been reported to have the ability to bind to structural proteins (Son-de Fernex et al., 2016) and depending on where and how they bind to nematode structures, they can inhibit egg hatching, development, larval motility and unsheathing (Hoste et al., 2012; Son-de Fernex et al. 2016).
In this sense, plants such as Gliricidia sepium, Leucaena leucocephala, Guazuma ulmifolia and Bursera simaruba are considered an important group of candidate plants with potential as food for animals in tropical and subtropical environments. In particular, Leucaena leucocephala and Gliricidia sepium are highly nutritious plants, legumes and rich in secondary metabolites (Son-de Fernex et al., 2012; Rivero-Pérez et al., 2019). They have been considered for their medicinal and anthelmintic properties (Sánchez and Faria, 2013; Canul-Solís et al., 2018), although they have not been consistently observed in animals.
On the contrary, Guazuma ulmifolia and Bursera simaruba have been little studied in order to examine their possible anthelmintic properties in animals, although in traditional medicine they are used against gastrointestinal and microbial diseases (Boligon et al., 2013); however, they are highly nutritious and widely used as feed or supplementary feed for livestock in tropical areas.
Therefore, the objective of this preliminary study was to determine the in vitro efficiency of Gliricidia sepium, Leucaena leucocephala, Guazuma ulmifolia and Bursera simaruba aqueous extracts at three doses (0.75, 1.00 and 1.25 mL), on the inhibition of the hatching of ovine gastrointestinal nematode eggs.
MATERIAL AND METHODS
Location
The study was carried out at the Animal Science Laboratory of the Postgraduate College, Campus Campeche, located at km 17.5 Haltunchén-Edzná highway, Champotón, Campeche, Mexico. Located at 19 ° 29 '51.79 "LN and 90 ° 32' 45.01" LO, with an altitude of 24 meters above sea level. The predominant climate is warm subhumid with rains in summer, with an average annual temperature of 26 °C (García, 2004).
Obtaining the aqueous extract
Gliricidia sepium, Leucaena leucocephala, Guazuma ulmifolia and Bursera simaruba plants were selected, with green leaves (young and mature), from which 1 kg of each was collected. The leaves were washed twice with purified water to remove dust and residues; then they were cut into 2 cm pieces and placed in 10 L plastic buckets, to which 1 L of distilled water was added, leaving it to stand for 12 h. After standing, the content of each cuvette was poured into 3 L aluminum containers and placed on heating racks at 80 °C for 40 min; then, it was ground with an immersion blender (T-fal®) for 5 min, to later filter it three times and deposit it in 300 mL containers, previously labeled for treatment. Finally, they were refrigerated at 5 °C until use (Vinueza et al., 2006).
The suspension obtained was considered as a standard solution (100%). From this solution, the doses were taken: 0.75, 1.00 and 1.25 mL, respectively for each treatment, plus a control group composed of distilled water, with 10 repetitions for each combination, as shown in table 1.
Table 1 Distribution of treatments to evaluate the efficiency of forage plant aqueous extracts at three doses in inhibiting the hatching of sheep gastrointestinal nematodes
| Dose (mL) Aqueous extract | 0.75 | 1.00 | 1.25 |
|---|---|---|---|
| Gliricidia sepium | 10 | 10 | 10 |
| Leucaena Leucocephala | 10 | 10 | 10 |
| Guazuma ulmifolia | 10 | 10 | 10 |
| Bursera simaruba | 10 | 10 | 10 |
| Distilled water (Control) | 10 | 10 | 10 |
For each combination of extract: dose, the content of total phenols (Folin), total tannins (Foli+pvpp) (Makkar et al., 1993) and condensed tannins (Vanillin) (Makkar and Becker, 1993) were determined as a reference, at the Faculty of Veterinary Medicine and Zootechnics of the Autonomous University of Yucatán, Yucatán, México (Table 2).
Table 2 Chemical analysis of total phenols, total tannins and condensed tannins of aqueous extracts from four forage plants
| Aqueous extract | Dose mL | Total phenols mg | Total tanninsmg | Condensed tannins mg |
|---|---|---|---|---|
| Gliricidia sepium | 0.75 | 0.73 | 0.39 | 0.67 |
| 1.00 | 0.97 | 0.52 | 0.89 | |
| 1.25 | 1.21 | 0.65 | 1.11 | |
| Leucaena leucocephala | 0.75 | 1.99 | 0.86 | 1.17 |
| 1.00 | 2.65 | 1.1 | 1.56 | |
| 1.25 | 3.31 | 1.44 | 1.95 | |
| Guazuma ulmifolia | 0.75 | 1.00 | 0.77 | 0.19 |
| 1.00 | 1.33 | 1.03 | 0.25 | |
| 1.25 | 1.66 | 1.29 | 0.31 | |
| Bursera simaruba | 0.75 | 1.35 | 0.59 | 0.70 |
| 1.00 | 1.8 | 0.78 | 0.93 | |
| 1.25 | 2.25 | 0.98 | 1.16 |
Obtaining feces and parasite load
The study was carried out according to the standards of use and care of animals destined for research of the Postgraduate College, Mexico and according to the Official Mexican Standard NOM-024-ZOO-1995.
Prior to the in vitro test, the number of eggs per gram of feces (e.p g.) was determined by means of a stool study. Sheep feces were obtained from a herd belonging to the “Los Robles” ranch located in Adolfo López Mateos, Escárcega, Campeche, Mexico, located at 18° 38' 09.51" NL and 90° 18' 04.69" LO, with a warm subhumid climate with rains in summer and average temperature of 26 °C (García, 2004). From which 60 sheep with an average age of 1.5 years were randomly taken, managed under a semi-stable system with day grazing, night confinement and without deworming in the eight months prior to collection.
All stool samples were homogenized in a single sample and processed using the McMaster technique, modified by Rodríguez-Vivas and Cob-Galera (2005), to count the number of GIN eggs, obtaining an average of 671.6 ± 250.4 epg, classified within a moderate infestation, ranging from 200 to 800 epg, recommended to establish parasite control (Morales et al., 2010).
Identification and proportion of GIN larvae genus
A larval culture was carried out with the previously described feces, with a duration of nine days, following the methodology of Corticelli and Lai (1963) described by (Niec, 1968), in order to determine the efficiency of the aqueous extracts on the hatching of GIN eggs; where the extracts at corresponding doses were applied every day at the time of aeration. The liquid collected in Falcon® tubes from the final phase of the larval culture was centrifuged (Centrifuge, VELAB VE-4000®) at 1500 rpm (415.8 x g) for 15 min, to collect the larvae by sedimentation.
Subsequently, they were placed in refrigeration at 5 ° C for five hours to stop metabolism and be counted by means of a stereoscopic microscope (VELAB VE-S3®). Of the total larvae obtained from each treatment, 100 were taken, to which 5% Lugol solution was added and observed with a microscope (UOP UB102i®), to be identified by morphological structures, based on the forelimb and/or mainly later (Niec, 1968).
Efficiency in inhibiting egg hatching
It was determined using equation (1) proposed by Álvarez et al. (2007).
Where, IEH = Percentage of efficiency in the inhibition of eggs, µTr= Arithmetic mean of the treated group and, µT= Arithmetic mean of the control group.
Statistical analysis
A completely randomized design with factorial arrangement (5 x 3) plus was used, where the plus was the control treatment. The factors were: Aqueous extract (G. sepium, L. leucocephala, G. ulmifolia, B. simaruba and Distilled water) and Dose (0.75, 1.00 and 1.25 mL). Data analysis was performed using Chi-square tests on the variables expressed in counts (%) and analysis of variance by the general linear models procedure (PROC GLM), of the SAS/STAT statistical package (SAS Institute Inc, 2012) in numeric variables. Comparisons of means were made by the Tukey test. All analyzes were carried out with a level of significance α = 0.05.
RESULTS AND DISCUSSION
Efficiency in inhibiting GIN eggs
Significant differences (p ≤ 0.05) were found in the average of hatched eggs, being the treatments with aqueous extracts of plants those that obtained a reduction of half of hatched eggs, compared to the control. The 1.25 mL dose obtained a greater (p ≤ 0.05) reduction in egg hatching, compared to the control. The smallest (p ≤ 0.05) egg hatchings were presented with extracts of L. leucocephala, G. sepium with doses at 1.25 mL and B. simaruba at 0.75 mL (Figure 1).
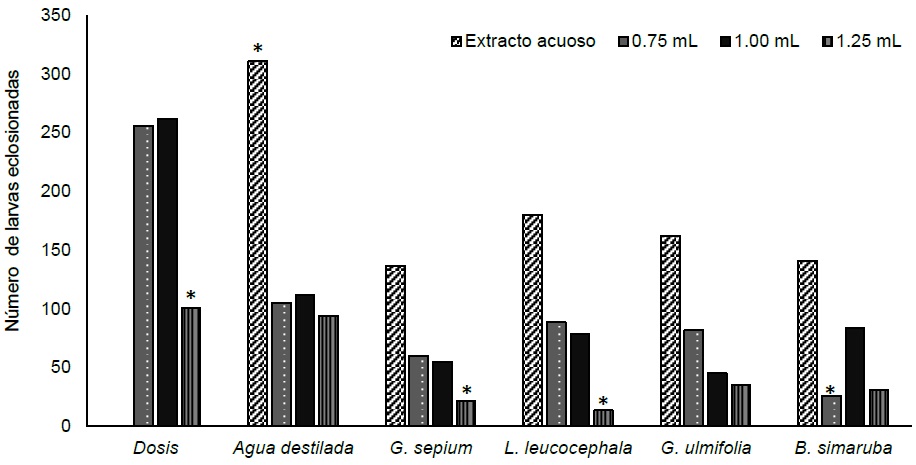
Figure 1 Hatched larvae average of sheep gastrointestinal nematodes with the addition of aqueous extracts of forage plants at three doses
The results found showed that the aqueous extracts actively affect the hatching of gastrointestinal nematodes eggs. This suggests that the activity of these plants is generally associated with the presence of secondary metabolites (Torres-Acosta et al., 2016; Oliveira et al., 2017), mainly condensed tannin concentration, without ruling out the participation of other secondary metabolites in the anthelmintic effect. It is alluded that the action mechanism of active compounds may be by interacting with cell membranes, which results in destabilization and the consequent increase in cell permeability that facilitates the action on egg intracellular proteins (Vieira et al., 2018) which inhibits its hatching.
However, other mechanisms may contribute to the observed effect, such as that reported by Vargas-Magaña et al. (2014) and Chan-Pérez et al. (2016), who mention that plant extracts can possibly inhibit enzymes´ reaction present in the egg membrane necessary for hatching, or preventing the formation of larvae by affecting the morula so that the larvae do not develop fully and do not achieve hatch. This results in a reduction in the number of larvae that hatch from the eggs; although, these are only hypotheses that should be tested in future studies.
Regarding IEH, the aqueous extracts were similar (p> 0.05) in effectiveness, with around 50% inhibition. The 1.25 mL dose was more efficient (p ≤ 0.05) compared to the rest of the doses evaluated. The combination L. leucocephala with doses of 1.25 mL was the most efficient (p ≤ 0.05), followed by G. sepium at 1.25 mL and B. simaruba at 0.75 mL.
It should be noted that, although the efficiency of the G. ulmifolia extract was not the best, it presented efficiencies greater than 59.8% with the 1.00 and 1.25 mL doses (Table 3).
Table 3 Efficiency of forage plant aqueous extracts at three doses on the inhibition of ovine gastrointestinal nematode eggs
| Aqueous extract | Dose (mL) | Efficiency (%) | Efficiency according to aqueous extract (%) |
|---|---|---|---|
| 0.75 | 48.8 cd | ||
| Gliricidia sepium | 1.00 | 51.2 d | 56.0 A |
| 1.25 | 77.6 g | ||
| 0.75 | 16.0 ab | ||
| Leucaena leucocephala | 1.00 | 30.0 bc | 42.0 A |
| 1.25 | 85.7 h | ||
| 0.75 | 22.0 abc | ||
| Guazuma ulmifolia | 1.00 | 59.8 de | 48.0 A |
| 1.25 | 62.4 ef | ||
| 0.75 | 75.4 g | ||
| Bursera simaruba | 1.00 | 25.1 abc | 55.0 A |
| 1.25 | 66.7 ef | ||
| 0.75 | 0.0 a | ||
| Distilled water | 1.00 | 0.0 a | 0.0 B |
| 1.25 | 0.0 a | ||
| 0.75 | 18.0 X | ||
| Efficiency according to dose | 1.00 | 16.0 X | |
| (%) 1.25 | 68.0 Y |
a, b, c, d, e, f, g, h. Different letter within each column indicates statistical difference (p ≤ 0.05). A, B. Different letter within each column indicates significant difference (p ≤ 0.05). X, Y. Different letter within each column indicates significant difference (p ≤ 0.05).
All the extracts were efficient in inhibiting the hatching of GIN eggs, compared to the control; possibly due to the presence of secondary metabolites present in the leaves (Martínez-Ortíz et al., 2013; Oliveira et al., 2017), mainly the condensed tannins to which a large part of this activity has been attributed; which in contact with the egg penetrate the cuticle, causing ultrastructural damage, preventing the development of the egg or paralyzing the larvae of the first stage (Vargas-Magaña et al., 2014). In some tropical legumes, in vitro anthelmintic activity has been reported against nematodes, suggesting that the activity may be related to the presence of tannins (Hoste et al., 2012; González- Cortázar et al., 2018).
On the other hand, non-legume plants, such as G. ulmifolia, their activity may be related to phenolic and flavonoid components (Feltrin et al., 2012), which have been reported with anthelmintic activity, which may suppose that these had some influence on the answer found for this excerpt. However, since the extract is a mixture of bioactive compounds, these can be acting individually, additively or synergistically.
The extracts of G. sepium, L. leucocephala, at a dose of 1.25 mL and B. simaruba with a dose of 0.75 mL inhibited larval hatching by 79%, this response is related to the higher content of condensed tannins, previously reported for these plants (Table 2), which may help explain the effects on IEH with these extracts. These results are higher than those reported by Puerto et al. (2014), who determined the in vitro effect on egg hatching, using the aqueous extract of G. sepium, obtaining 40% inhibition and 50% inhibition, with doses of 7.90 mg mL-1 using L. leucocephala reported by Son-de Fernex et al. (2016), with aqueous and acetonic extracts, but not for G. sepium, obtaining a 100% inhibition of the eggs at a concentration of 1.03 mg mL-1.
In general, a positive dose-dependent effect was observed in most of the plant extracts; however, in the extract based on B. simaruba, the greatest responses were found at lower doses, so it is proposed that the secondary metabolites of this plant have a more defined action on this activity, making it a good candidate for future investigations.
The plant extracts used have a positive response towards the control of the hatching of GIN eggs by means of the phytochemicals present. However, more studies are required on the identification of the molecules present in the extracts, this would help to understand action mechanisms involved in their effects on the GIN.
Identification and proportion of genus of GIN larvae
Five genera of GIN larvae were identified, being Haemonchus spp., the one with the highest (p ≤ 0.05) prevalence with 58.0%, continuing Trichostrongylus spp., and Oesophagostomum spp., with 25.0 and 15.0%, respectively. The genera Cooperia spp., and Nematodirus spp., achieved 2.0% prevalence. Table 4 shows the most effective combinations in the inhibition (p ≤ 0.05) of eggs, according to the genus of GIN.
Table 4 Hatched larvae according to genus of sheep gastrointestinal nematodes with the addition of forage plant aqueous extracts at three doses
| Genera of larvae (%) | ||||||
|---|---|---|---|---|---|---|
| Aqueous extract | Dose (mL) | Haemonchus spp. | Oesophagostomum spp. | Trichostrongylus spp. | Cooperia spp. | Nematodirus spp. |
| 0.75 | 60.2 | 77.2 | 27.6 | 0.0* | 0.0 | |
| G. sepium | 1.00 | 48.7 | 38.3 | 57.7 | 50.0 | 0.0 |
| 1.25 | 29.9* | 26.6* | 15.7* | 3.8 | 0.1 | |
| 0.75 | 80.9 | 60.5 | 100.0 | 100.0 | 0.0 | |
| L. leucocephala | 1.00 | 96.8 | 19.1* | 50.4 | 33.3 | 0.0 |
| 1.25 | 16.8* | 9.4* | 15.2* | 3.8* | 0.0 | |
| 0.75 | 100.0 | 13.2* | 55.2 | 100.0 | 0.0 | |
| G. ulmifolia | 1.00 | 55.7 | 15.7* | 25.2* | 0.0* | 0.0 |
| 1.25 | 39.6* | 6.3* | 44.9 | 53.8 | 0.1 | |
| 0.75 | 21.4* | 28.9* | 27.6 | 100.0 | 0.0 | |
| B. simaruba | 1.00 | 54.1 | 57.4 | 100.0 | 50.0 | 0.0 |
| 1.25 | 41.1 | 31.3 | 24.2* | 46.2* | 0.1 | |
| 0.75 | 100.0 | 100.0 | 100.0 | 100.0 | 0.0 | |
| Distilled water | 1.00 | 100.0 | 100.0 | 100.0 | 100.0 | 0.0 |
| 1.25 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |
*Indicates statistical difference (p ≤ 0.05) within each column
It is observed that the action of the aqueous extracts on the genera found was positive, since there is a decrease in the hatching of eggs. The extracts of G. sepium and L. leucocephala at 1.25 mL have a greater inhibitory effect on four of the five genera found; while for Cooperia spp., at lower doses of G. sepium and G. ulmifolia they obtained a greater effect. In this sense, it has been reported that for the Cooperia spp genus, phenolic compounds and flavonoids have shown activity (Son-de Fernex et al., 2015), which suggests that the phenolic compounds in these extracts may have an important role in the inhibition of this genus.
The aqueous extracts inhibited the hatching of Haemonchus contortuseggs by 46%, which is a highly pathogenic helminth of small ruminants, with global distribution. The most efficient combinations were L. leucocephala, G. sepium at 1.25 mL and B. simaruba at 0.75 mL with approximately 77% efficiency, the result being related to the content of condensed tannins present. As mentioned by Vargas-Magaña et al. (2014), who point out that the anthelmintic activity of plant extracts on Haemonchus contortus eggs has been mainly related to the content of condensed tannins and other secondary metabolites that contribute partially or totally. They have been speculated to include alkaloids, saponins, phenolic compounds (Ferreira et al., 2013), and more recently monoterpenoids (Goel et al., 2020).
It is important to highlight the extract of B. simaruba, which at lower doses obtained inhibitions greater than 70% in Haemonchus spp., Oesophagostomum spp., and Trichostrongylus spp., so it is a source of research.
The action of condensed tannins and other secondary plant compounds are not exactly known and can vary with the parasite, its developmental stage, and the biochemical characteristics of the plant species. However, it has been identified that the outer membrane of GIN eggs and larvae is rich in lipids and glycoproteins where tannins can bind, resulting in the accumulation of aggregates (Hoste et al., 2006) that can affect development of the larvae, decreasing the hatching of the egg or its motility (Martínez- Ortíz et al., 2013).
The efficacy obtained in the present study provides us with useful information that allows us a starting point for studies focused on determining optimal doses, identification and isolation of molecules with anthelmintic activity, present in the extracts with the highest bioactivity in the control of the hatching of GIN.
CONCLUSIONS
The aqueous extracts of Gliricidia sepium, Leucaena leucocephala, Guazuma ulmifolia and Bursera simaruba exhibited anthelmintic activity against GIN eggs. However, the 1.25 mL dose and Leucaena leucocephala and Gliricidia sepium extracts at 1.25 mL doses were the most effective. These preliminary results could be a possible sustainable alternative for the prevention and parasitosis control in hair sheep, highlighting the need for studies on the identification of the bioactive compounds responsible for this activity.
ACKNOWLEDGMENT
To the National Council of Science and Technology (CONACyT) for the scholarship awarded to the first author. To Cátedras CONACyT 2181 "Agroecological strategies for food security in rural areas of Campeche" project fomr the Postgraduate College Campeche campus and to the owner of the Ranch "Los Robles", Mr. Marcos Gamboa.
REFERENCES
Álvarez CV, Hernández J, Wing CR. 2007. Eficacia de aserrines para inhibir el desarrollo in vitro de larvas de parásitos gastrointestinales de ovinos. Agronomía Costarricense. 31(1):71-75. ISSN: 0377-9424. http://hdl.handle.net/10669/13815 [ Links ]
Boligon AA, Feltrin AC, Athayde ML. 2013. Determination of chemical composition, antioxidant and antimicrobial properties of Guazuma ulmifolia essential oil. American Journal of Essential Oils and Natural Products. 1(1):23-27. ISSN: 2321-9114. https://www.essencejournal.com/vol1/issue1/pdf/7.1.pdf [ Links ]
Canul-Solís J, Alvarado-Cánche C, Castillo-Sánchez L, Sandoval-Gío J, Alayón-Gamboa J, Peñeiro-Vázquez A, Chay-Canul A, Casanova-Lugo F, Ku-Vera J. 2018. Gliricidia sepium (Jacq.) Kunth ex Walp. Una especie arbórea multipropósito para la sustentabilidad de los agroecosistemas tropicales. Agroproductividad. 11(10):195-200. ISSN: 2594-0252. https://doi.org/10.32854/agrop.v11i10.1268 [ Links ]
Chan-Pérez JI, Torres-Acosta JFJ, Sandoval-Castro CA, Hoste H, Castañeda-Ramírez GS, Vilarem G, Mathieu C. 2016. In vitro susceptibility of ten Haemonchus contortus isolates fron different geographical origins towards acetone:wáter extracts of two tannin rich plants. Veterinary Parasitology. 217:53-60. ISSN: 0304-4017. https://doi.org/10.1016/j.vetpar.2015.11.001 [ Links ]
Feltrin AC, Boligon AA, Janovik V, Athayde ML. 2012. Antioxidant potential, total phenolic and flavonoid contents from the stem bark of Guazuma ulmifolia Lam. Asian Journal of Biological Sciences. 5(5):268-272. ISSN: 1996-3351. https://doi.org/10.3923/ajbs.2012.268.272 [ Links ]
Ferreira LE, Castro PMN, Chagas ACS, Franca SC, Beleboni RO. 2013. In vitro anthelmintic activity of aqueous lef extract of Annona muricata L. (Annonaceae) against Haemonchus contortus from sheep. Experimental Parasitology. 134:327-332. ISSN: 0014-4894. https://doi.org/10.1016/j.vetpar.2018.02.016 [ Links ]
García E. 2004. Modificaciones al Sistema de Clasificación Climática de Köppen (para adaptarlo a las condiciones de la República Mexicana). Quinta edición. Instituto de Geografía/UNAM. México. Pp. 90. ISBN: 970-32-1010-4. https://www.academia.edu/12911044/Modificaciones_al_sistema_de_clasificaci%C3%B3n_clim%C3%A1tica_de_K%C3%B6ppen_para_adaptarlo_a_las_condiciones_de_la_Rep%C3%BAblica_Mexicana_2004_Enriqueta_Garc%C3%ADa [ Links ]
Goel V, Das Singla L, Choudhury D. 2020.Cuminaldehyde induces oxidative stress- mediated physical damage and death of Haemonchus contortus. Biomedicine & Paharmacotherapy. 130:110411. ISSN: 0753-3322. https://doi.org/10.1016/j.biopha.2020.110411 [ Links ]
González-Cortázar M, Zamilpa A, López-Arellano ME, Aguilar-Marcelino L, Reyes-Guerrero DE, Olazarán-Jenkins S, Ramírez-Vargas G, Olmedo-Juárez A, Mendoza- de Gives P. 2018. Lysiloma acapulcensis leaves contain anthelmintic metabolites that reduce the gastrointestinal nematode egg population in sheep faeces. Comparative Clinical Pathology. 27:189-197. ISSN: 1618-565X. https://doi.org/10.1007/s00580-017-2577-1 [ Links ]
Holsback L, Ramsey LPA, Sanches SC, Kremer NG, Conde G, Vinícius GH, Balestrieri JV, Tomazella L. 2016. Anthelmintic efficiency of doramectin, fenbendazole, and nitroxynil, in combination or individually, in sheep worm control. Revista Brasileira de Parasitologia Veterinária. 23(3):353-358. ISSN: 1984-2961. http://dx.doi.org/10.1590/S1984-29612016025. [ Links ]
Hoste H, Martínez-Ortiz-De-Montellano C, Manolaraki F, Brunet S, Ojeda-Robertos N, Fourquaux I, Torres-Acosta JFJ, Sandoval-Castro CA. 2012. Direct and indirect effects of bioactive tannin-rinch tropical and temperate legumes against nematode infections. Veterinary Parasitology. 186 (1-2):18-27. ISSN: 0304-4017. https://doi.org/10.1016/j.vetpar.2011.11.042 [ Links ]
Hoste H, Jackson F, Athanasiadou S, Thamsborg SM, Hoskin SO. 2006. The effects of tannin-rich plants on parasitic nematodes in ruminants. TRENDS in Parasitology. 22(6):253-261. ISSN: 1471-4922. https://doi.org/10.1016/j.pt.2006.04.004 [ Links ]
Makkar HPS, Becker K. 1993. Vanillin-HCl method for condensed tannins: effect of organic solvents used for extraction of tannins. Journal of Chemical Ecology. 19:613-621. ISSN: 1573-1561. http://doi.org/10.1007/BF00984996 [ Links ]
Makkar HPS, Blummel M, Borowy NK, Becker K. 1993. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. Jornal of the Science of Food and Agriculture. 61:161-165. ISSN: 1097-0010. http://doi.org/10.1002/jsfa.2740610205 [ Links ]
Martínez-Ortíz de MC, Arroyo-López C, Fourquaux I, Torres-Acosta JFJ, Sandoval-Castro CA, Hoste H. 2013. Scanning electron microscopy of Haemonchus contortus exposed to tannin-rich plants under in vivo and in vitro conditions. Experimental Parasitology. 133(3):281-286. ISSN: 0014-4894. https://doi.org/10.1016/j.exppara.2012.11.024 [ Links ]
Medina P, Guevara F, La OM, Ojeda N, Reyes E. 2014. Resistencia antihelmíntica en ovinos: una revisión de informes del sureste de México y alternativas disponibles para el control de nemátodos gastrointestinales. Pastos y Forrajes. 37(3):257-263. ISSN: 0864-0394. http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0864-03942014000300001 [ Links ]
Mondragón-Ancelmo J, Olmedo-Juárez A, Reyes-Guerrero DE, Ramírez-Vargas G, Ariza-Román AE, López-Arellano ME, Gives PM, Napolitano F. 2019. Detection of Gastrointestinal Nematode Populations Resistant to Albendazole and Ivermectin in Sheep. Animals. 9(10):775. ISSN: 2076-2615. http://doi.org/10.3390/ani9100775 [ Links ]
Morales G, Guillen TA, Pinho A, Pino L, Barrios F. 2010.Clasificación por el método Famacha y su relación con el valor de hematocrito y recuento de H.P.G. de ovinos criados en condiciones de pastoreo. Zootecnia Tropical. 28(4):545-556. ISSN: 0798-7269. http://www.scielo.org.ve/pdf/zt/v28n4/art11.pdf [ Links ]
Niec R. 1968. Cultivo e identificación de larvas infectantes de nematodos gastroentéricos del bovino y ovino. Manual Técnico 3. Instituto Nacional de Tecnología Agropecuaria (INTA).Buenos Aires, Argentina. Pp. 37. http://helminto.inta.gob.ar/Niec/Cultivo%20e%20Identificaci%C3%B3n%20de%20Larvas%20Infectantes%20de.pdf [ Links ]
NORMA Oficial Mexicana. NOM-024-ZOO-1995. Especificaciones y características zoosanitarias para el transporte de animales, sus productos y subproductos, productos químicos, farmacéuticos, biológicos y alimenticios para uso en animales o consumo por éstos. http://www.gob.mx/cms/uploads/attachment/file/202301/NOM-024-ZOO- 1995_161095.pdf [ Links ]
Oliveira FA, Costa JML, Lima SA, Silva RC, Ribeiro NSM, Mesquista WCJ, Rocha QC, Tangerina MPM, Villegas W. 2017. Anthelmintic activity of plant extracts from Brazilian savanna. Veterinary Parasitology. 236:121-127. ISSN: 0304-4017. https://doi.org/10.1016/j.vetpar.2017.02.005 [ Links ]
Puerto AM, Arece GJ, López LY, Roche Y, Molina M, Sanavria A, da Fonseca AH. 2014. Efecto in vitro de extractos acuosos de Moringa oleifera y Gliricida sepium en el desarrollo de las fases exógenas de estrongílidos gastrointestinales de ovinos. Revista de Salud Animal. 36(1):28-34. ISSN: 0253-570X. http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0253-570X2014000100005 [ Links ]
Rivero-Pérez N, Jaramillo-Colmenero A, Peláez-Acero A, Rivas-Jacobo M, Ballesteros-Rodea G, Zaragoza-Bastida A. 2019. Actividad antihelmíntica de la vaina de Leucaena leucocephala sobre nemátodos gastrointestinales de ovinos (in vitro). Abanico Veterinario. 9(95):1-9. ISSN: 2448-6132. https://doi.org/10.21929/abavet2019.95. [ Links ]
Rodríguez-Martínez R, Mendoza-de-Gives P, Aguilar-Marcelino L, López- Arellano ME, Gamboa-Angulo M, Rosas-Saito GH, Reyes-Estébanez M, García-Rubio VG. 2018. In vitro lethal activity of the nematophagous fungus Clonostachys rosea (Ascomycota: Hypocreales) against nematodes of five different taxa. BioMed Research International 2018:3501827. ISSN: 2314-6133. https://doi.org/10.1155/2018/3501827 [ Links ]
Rodríguez-Vivas RI, Cob-Galera, LA. 2005. Técnicas Diagnósticas en Parasitología Veterinaria. Terceraedición. 2da. Edición. Ediciones de la Universidad Autónoma de Yucatán. Mérida, México. Pp. 41-51. ISBN: 9709680458, http://bibliotecasibe.ecosur.mx/sibe/book/000049652 [ Links ]
Rodríguez-Vivas RI, Grisi L, Pérez LAA, Silva VH, Torres-Acosta JFJ, Sánchez FH, Romero SD, Rosario RC, Saldierna F, García CD. 2017. Potential economic impact assessment for cattle parasites in México. Review. Revista Mexicana de Ciencias Pecuarias. 8(1):61-74. ISSN: 2448-6698. http://dx.doi.org/10.22319/rmcp.v8i1.4305 [ Links ]
Sánchez A, Faria MJ. 2013. Efecto de la madurez de la planta en el contenido de nutrientes y la digestibilidad en una asociación Cenchrus ciliaris-Leucaena leucocephala. Zootecnia Tropical. 31(1):16-23. ISSN: 0798-7269. http://usfx.bo/nueva/vicerrectorado/citas/AGRARIAS_7/Zootecnia/Gutierrez,%20A.%20J%20S.%20(2014).%20Efecto%20de%20la.pdf [ Links ]
SAS Institute. 2012. Statistical Analysis Software SAS/STAT®, versión 9.0.2, Cary, North Carolina, USA: SAS Institute Inc., ISBN: 978-1-60764-599-3. http://www.sas.com/en_us/software/analytics/stat.html# [ Links ]
Son-De Fernex EV, Alonso DMA, Mendoza GP, Valles MB, Zamilpa A, González CM. 2016. Ovicidal activity of extracts from four plant species against the cattle nematode Cooperia punctata. Veterinaria México OA. 3(2):1-14. ISSN: 2448-6760. https://doi.org/10.21753/vmoa.3.2.365 [ Links ]
Son-De Fernex EV, Alonso-Díaz MA, Mendoza-de Gives P, Valles-de la Mora B, González-Cortazar M, Zamilpa A, Castillo Gallegos E. 2015. Elucidation of Leucaena leucocephala anthelmintic-like phytochemicals and the ultrastructural damage generated to eggs of Cooperia spp. Veterinay Parasitology. 214:89-95. ISSN: 0304-4017. https://doi.org/10.1016/j.vetpar.2015.10.005 [ Links ]
Son-De Fernex EV, Alonso-Díaz MA., Valles-de la Mora B, Capetillo-Leal CM. 2012. In vitro anthelmintic activity of five tropical legumes en the exsheathment and motility of Haemonchus contortus infective larvae. Experimental Parasitology. 131:413-418. ISSN: 0014-4894. https://doi.org/10.1016/j.exppara.2012.05.010 [ Links ]
Torres-Acosta JFJ, González-Pech PG, Ortiz-Ocampo GI, Rodríguez-Vivas I, Tun-Garrido J, Ventura-Cordero J, Castañeda-Ramírez GS, Hernández-Bolio GI, Sandoval-Castro CA, Chan-Pérez JI, Ortega-Pacheco A. 2016. Revalorización del bosque tropical caducifolio para la producción de rumiantes. Tropical and Subtropical Agroecosystems. 19:73-80. ISSN: 1870-0462. http://www.redalyc.org/articulo.oa?id=93945700009 [ Links ]
Vargas-Magaña JJ, Torres-Acosta JFJ, Aguilar-Caballero AJ, Sandoval-Castro CA, Hoste H, Chan-Pérez JI. 2014. Anthelmintic activity of acetone-water extracts against Haemonchus contortus eggs: interactions between tannins and other plant secondary compounds. Veterinary Parasitology. 206:322-327. ISSN: 0304-4017. https://doi.org/10.1016/j.vetpar.2014.10.008 [ Links ]
Vieira SAC, Oliveira SF, Goncalves LH, Dias DSG, Soares UR., Reis DÊ, Branco A, Viana CK, Mauricio DJ, Borges BM, Lima CS, Moreira BMJ. 2018. In vitro ovicidal and larvicidal activities of some saponins and flavonoids against parasitic nematodes of goats. Parasitology. 145(14):1884-1889. ISSN: 1469-8161. http//doi.org/10.1017/S0031182018000689 [ Links ]
Vinueza S, Crozzoli R, Perichi G. 2006. Evaluación in vitro de extractos acuosos de plantas para el control del nemátodo agallador Meloidogyne incognita. Fitopatología de Venezuela. 19:26-31. ISSN: 0798-0035. https://www.researchgate.net/publication/48224786 [ Links ]
Received: September 09, 2020; Accepted: February 02, 2021; Published: February 15, 2021











 texto en
texto en 



