Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Abanico veterinario
versão On-line ISSN 2448-6132versão impressa ISSN 2007-428X
Abanico vet vol.11 Tepic Jan./Dez. 2021 Epub 11-Out-2021
https://doi.org/10.21929/abavet2021.20
Original Article
Climatic, regional, and bee brood quantity influences on the hygienic behavior of Apis mellifera
¹Departamento de Ciencias Económicas y Administrativas. Centro Universitario del Sur. Universidad de Guadalajara. México, C.P. 49000.
²Departamento de Producción Agrícola, Centro Universitario de la Costa Sur. Universidad de Guadalajara. México, C.P. 48900.
3School of Environmental Sciences, University of Guelph, 50 Stone Road East, Guelph, N1G 2W1, Ontario, Canada.
4 Centro en Investigaciones en Abejas, Centro Universitario del Sur. Universidad de Guadalajara. México, C.P. 49000.
The hygienic behavior of honeybees (Apis mellífera) is a mechanism highly influenced by genetic effects that confers resistance against diseases and parasites. The objective of this study was to determine whether climatic, regional and brood quantity influences are related to hygienic behavior in beehives in Jalisco state, Mexico. Freezing pupae with liquid nitrogen in two climatic zones (mountain and valley) evaluated 142 colonies. Colonies located in the municipalities of the low zone removed significantly more frozen brood than the colonies located in the municipalities of the higher zone (p <0.001), and these differences were significantly correlated with environmental temperature (r = 0.25, p <0.01) and altitude above sea level (r =- 0.27, p <0.001). It was also found that in the warmer, lower altitude region, one third of the colonies were highly hygienic (> 80 %). This degree of cleanliness is acceptable for initiating selective breeding projects for highly hygienic bees to improve colony health, which would benefit the beekeeping industry.
Keywords: Apis mellífera; hygienic behavior; reproduction; climate; altitude
El comportamiento higiénico de las abejas (Apis mellífera) es un mecanismo altamente influenciado por efectos genéticos que les confiere resistencia contra enfermedades y parásitos. El objetivo de este estudio fue determinar si las influencias climáticas, regionales y de cantidad de cría se relacionan con el comportamiento higiénico en colmenas del estado de Jalisco, México. Se evaluaron 142 colonias mediante el método de congelación de pupas con nitrógeno líquido en dos zonas climáticas (zonas montaña y valle). Las colonias ubicadas en los municipios de la zona baja removieron significativamente más cría congelada que las colonias ubicadas en los municipios de la zona alta (p < 0.001) y estas diferencias estuvieron significativamente correlacionadas con la temperatura ambiental (r = 0.25, p < 0.01) y la altura sobre el nivel del mar (asnm) (r = - 0.27, p < 0.001). También se encontró que en la región más cálida y de menor altitud, un tercio de las colonias fueron altamente higiénicas (> 80%). Este grado de limpieza es aceptable para iniciar proyectos de crianza selectiva de abejas altamente higiénicas para mejorar la sanidad de las colonias, lo cual beneficiaría a la industria apícola.
Palabras Clave: Apis mellífera; comportamiento higiénico; reproducción; clima; altitud
INTRODUCTION
Mexico is one of the main producing and exporting countries of bees (Apis Mellifera) in the world and beekeeping is an activity of enormous socio-economic importance for the country. About 45,000 producers are dedicated to it, who work around 1.9 million colonies (Magaña et al., 2016). However, in recent years, honey production has not increased due to lack of processes that can potentialize this sector (Contreras-Escareño et al., 2013; Soto-Muciño et al., 2017). Mexican beekeeping is affected by various factors that reduce the productivity and profitability of colonies, including diseases of bees caused by various microorganisms and parasites (Magaña-Magaña et al., 2016). In Jalisco, one of the main honey-producing states of Mexico, prevail diseases that affect adult breeding and bees, such as nosemosis, varroosis and ascosfereosis (Tapia-González et al., 2017, 2019, 2020). These health problems can produce economic losses of many millions of pesos annually, but losses could be reduced if beekeepers establish selective breeding programs to develop strain-resistant bees and parasites.
One of resistance mechanisms to diseases and parasites of the best-known bees is the hygienic behavior, which consists of the ability of bees to detect dead, sick, or parasitized puppets and destructive mites, with the end to open the cells to clean them. Therefore, through this essential mechanism, pathogen transmission risks and parasites are reduced (Emsen et al., 2012). Hygienic behavior is important in social insects for overcrowding in which they live, since, if an individual gets sick, the possibility of transmission between members of the colony is very high (Guzman-Novoa and Morfin, 2019). In addition, bees colonies with high hygienic behavior can increase the production of honey (Wielewski et al., 2012).
Genetic effects affect the hygienic behavior of the honeybees and it is heredable (Arechavaleta-Velasco et al., 2011; Pernal et al., 2012), so it is viable to develop highly hygienic bees with genetic improvement programs (Ivernizzi et al., 2011). The expression of hygienic behavior is variable (Xonis et al., 2015), which is due in part to the aforementioned genetic effects, but also to the influence of environmental factors. Little is known about the environmental effects that affect the hygienic behavior of bees, but it has been reported that the amount of colony breeding and changes in the flow of nectar collected by bees can affect it (Wagoner et al., 2018). Other studies support the idea of bees’ behavior (Sousa et al., 2016). It is important to determine what environmental factors influence the hygienic behavior of the honeybees to standardize its effects when establishing selective breeding programs to develop highly hygienic bee’s types. In this sense, the objective of this study was to determine whether climate, regional influences are related to hygienic behavior in hives of Jalisco state, Mexico.
MATERIAL AND METHODS
Study regions. The present work was held in apiaries of seven municipalities of Jalisco state, Mexico, located in a high region or mountain (Tapalpa and Unión de Guadalupe) and in a low or level region (Tamazula de Gordiano, Zapotlán el Grande, Sayula, San Martín Hidalgo and Zacoalco de Torres). The climate and altitude above sea level (M A.S.L) present great contrasts due to the varied relief conformation of these regions (Regional Development Plan, 2012). To evaluate their hygienic behavior and reproductive capacity in the seven municipalities mentioned 142 colonies of 45 apiaries were used (Table1) during the dry period (between March and May).
Table 1 Number of colonies and apiaries of honeybees evaluated for hygienic behavior by
| Municipality | Region | No. colonies | No. apiaries |
|---|---|---|---|
| Tapalpa | Mountain | 27 | 9 |
| Unión de Guadalupe | Mountain | 28 | 9 |
| Tamazula | Plain | 17 | 5 |
| Zapotlán el Grande | Plain | 14 | 4 |
| Sayula | Plain | 22 | 7 |
| San Martin Hidalgo | Plain | 18 | 6 |
| Zacoalco | Plain | 16 | 5 |
| Total | Plain | 142 | 45 |
Experimental units. From each apiary of the seven municipalities were randomized and marked from three to four colonies, which were identified with a plastic album where the colony number was recorded and location records were taken. Each selected colony was opened and inspected to ensure that signs of breeding diseases were not detected visually (American foulbrood, European foulbrood, Chalkbrood, or Sacbrood). The colonies that presented any indication of illness were not used.
Evaluation of hygienic behavior. From each of the selected colonies, the breeding frame was chosen with as many cells operated with a continuous and uniform pattern. The chosen frame was placed horizontally up the hive, and on it, a cylinder was placed without a vinyl polychloride (PVC) of 10 x 11 cm, exerting slight pressure with movements in a circle over the operated breeding. It was estimated that this cylinder covered an average amount of 350 cells. Subsequently, the breeding was taken into slaughter within the cylinder by freezing (Spivak, 1996). For this, 250 ml of liquid nitrogen (-195 °C) was emptied inside this cylinder (-195 °C) using an aluminum pouring. Once the evaporation process of the nitrogen (approx 1 min) is finished, a digital image (Nikkon, Coolpix) image was taken from the frozen breeding area to later count the number of initial cells. The experimental frame was identified with the number of the colony, marking it with indelible down on the head. Subsequently, the frame was introduced to the center of the breeding chamber of the origin colony so that the workers will begin the removal of slaughtered brood (hygienic behavior). After 24 hours of this procedure, the hive was reopened and the frame was extracted with the brood that was frozen. A second photograph of the frozen area was taken to count the number of clean cells (where the bees removed pupae). Then, using cell numbers of both photographs, removal brood percentage (Espinosa-Montaño et al., 2008) was determined, this procedure was performed on one occasion. The following formula was used to obtain the percentage of brood removal:
Reproductive capacity determination of colonies. To determine the reproductive capacity, the number of racks with an capped brood was calculated in each colony by estimating the proportion of the area of each honeycomb which was covered with sealed breeding (0-1.0) and these values were added (Delaplane et al., 2013).
Determination of altitude and climatic variables. To measure the altitude above sea level (m a.s.l) of each apiary, a GPS (Sportrack-Color, Magellan) was used. The average temperature and ambient humidity data were obtained from the electronic page, municipalities of Jalisco (Jalisco State Goverment, 2019).
Statistical analysis. The percentage data of hygienic behavior were transformed into the square root arcosum to normalize them as suggest it William (1990) and Medina-Flores et al (2019). The number of honeycombs with breeding required logarithmic transformation and climate data were not transformed by presenting a normal distribution, to define the normality of the data; the Kolmogorov-Smirnov (Hanusz and Tarasińska, 2015) test was used. The hygienic responses of colonies, as well as the amount of breeding and climatic parameters were analyzed between municipalities by means of variance analysis. When significantly being found, the means were separated and compared with Fisher's protected tests (p <0.05). The data between regions were compared with Student T tests. In addition, with Pearson's tests, the hygienic response of the colonies with the amount of breeding and climate parameters was correlated. In addition, frequency distribution histograms were created in order to determine the percentage of colonies with low and high hygienic behavior for the colonies of each region. Analyzes were carried out with the statistical package SPSS Version 24 ® (Quezada- Lucio, 2017).
RESULTS
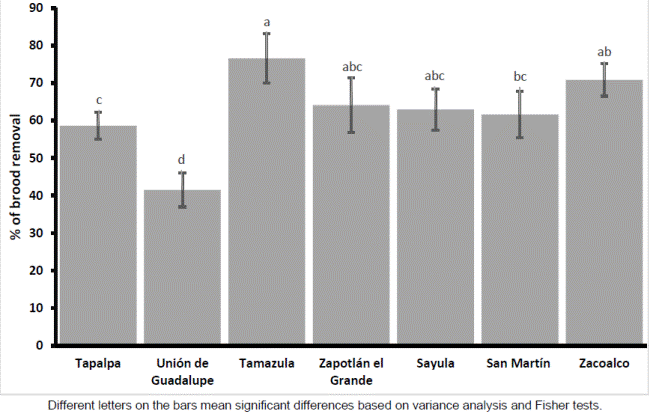
The degree of hygienic behavior of bees’ colonies varied significantly between municipalities (F6,135 = 4.88, p <0.001) and between regions (T140 = - 4.05, p <0.001). The colonies located in the municipalities of the plain region were significantly more hygienic than the colonies located in the Mountain Municipalities (Figures 1, 2).

Figure 2 Brood removal percentage ± S.E. of 142 bee colonies in two regions Different letters on the bars mean significant differences base don a student T test
Significant differences were found in the amount of colonies breeding, as well as in the relative temperature and humidity between municipalities (F6,135 = 4.94, F6,135 = 70.28, F6,135 = 63.07, p <0.0001, respectively). Significant differences were also observed for the temperature between region (T140 = - 10.50, p <0.0001), but not for relative humidity and amount of breeding (T140 = - 0.31 and T140 = - 0.32, p> 0.75, respectively). For breeding and relative humidity, a pattern related to the region or with the m a.s.l was not observed. There was similar variation in municipalities of both regions for these two factors. For the temperature factor, colonies located in municipalities of the plain area had a significantly warmer climate than colonies located in the mountain municipalities (Tables 2, 3).
Table 2 Mean ± S.E. of brood quantity (number of honeycombs) and climatic and environmental factors (height above sea level: m a.s.l) of bees colonies evaluated for hygienic behavior by municipality
| Municipality | Hives w/brood | Temperature | Relative humidity | m.a.s.l | |
|---|---|---|---|---|---|
| Tapalpa | 4.9 ± 0.2c | 29.2 ± 0.3c | 24.2 ± 0.6c | 2119 ± 22 | |
| Unión de Guadalupe | 5.7 ± 0.2a | 23.3 ± 0.6d | 34.6 ± 2.0b | 1912 ± 18 | |
| Tamazula | 6.1 ± 0.4a | 33.3 ± 0.4b | 16.5 ± 0.5d | 1313 ± 10 | |
| Zapotlán el Grande . | 5.0 ± 0.3bc | 27.9 ± 0.3c | 42.9 ± 1.3a | 1512 ± 21 | |
| Sayula | 5.5 ± 0.3abc | 36.5 ± 0.6a | 36.7 ± 1.1b | 1425 ± 38 | |
| San Martín Hidalgo | 4.3 ± 0.2c | 34.2 ± 0.7a,b | 13.8 ± 0.6e | 1317 ± 12 | |
| Zacoalco | 5.3 ± 0.2abc | 31.9 ± 0.9b | 44.1 ± 2.6a | 1455 ± 12 |
Different letters indicate significant differences between municipalities based on variance analysis and Fisher tests
Table 3 Mean ± S.E. of brood quantity (number of honeycombs) and climatic and environmental factors (height above sea level: m a.s.l) of bees colonies evaluated for hygienic behavior by region
| Region | Hives w/brood | Temperature | Relative humidity | m.a.s.l |
|---|---|---|---|---|
| Plain | 5.2 ± 0.1a | 33.2 ± 0.4a | 30.2 ± 1.5a | 1400 ± 13 |
| Mountain | 5.3 ± 0.2a | 26.2 ± 0.5b | 29.5 ± 1.3a | 2016 ± 20 |
Different letters indicate significant differences between regions based on Student T tests.
Significant correlations were found between the degree of hygienic behavior of colonies, ambient temperature and m a.s l. However, there was no significant correlation of the hygienic behavior of colonies or the amount of brood or relative humidity (Table 4).
Table 4 Correlations and trusted interval (C. I. 95%) between the level of hygienic behavior (H. B.) of bee’s colonies evaluated and climatic, environmental and honeycomb factors with breeding
| Correlated characteristics | r | p | C. I. 95% | |
|---|---|---|---|---|
| H. B. - Temperature | 0.25 | 0.002 | 0.09-0.40 | |
| H. B. - Relative Humidity | 0.02 | 0.826 | - 0.15-0.19 | |
| H. B. - Meters above sea level | - 0.27 | 0.001 | - 0.41-0.12 | |
| H. B. - Hives with brood | 0.03 | 0.756 | - 0.14-0.20 |
Analyzing all colonies evaluated, 50% of them had low levels of hygienic behavior (<60%), while 25.3 and 11.3% of them, had a high hygienic behavior (> 80 %) or very high (> 95 %) respectively. The analysis by regions revealed that, in the plain region, a higher percentage of colonies showed high hygienic behavior (> 80 %) compared to colonies of the mountain region (34.5 and 10.9 %, respectively, Figures 3A and 3B). In addition, in the plain region, 16.1% of the colonies showed a very high sanitary behavior (> 95 %) against only 3.6% of colonies in the mountain region. This study showed that the colonies of bees located in the municipalities of the plain region were significantly more hygienic than the colonies located in municipalities of the mountain region and that these differences are related to environmental temperature and m a.s.l. It was also found that in the warmest and lower altitude municipalities, there is a greater proportion of bees’ colonies with high or very high hygienic behavior than in colder and in high sites.
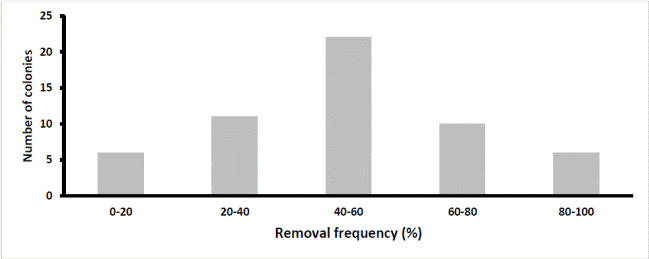
Figure 3a Frequency of colonies with different percentages of brood removal in the mountain area (n = 55)
DISCUSSION
Although there was a degree of variability between municipalities, the number of honeycombs with breeding of bee colonies was not related to brood removal, so it apparently did not influence the hygienic behavior in bee populations studied. This result was probably due to a reduced variation for breeding of the colonies studied between regions. It is possible that at another time of year these conditions could change and that differences of honeycombs with breeding in the colonies could result in population differences that would influence their degree of hygienic behavior. Regardless of that possible hypothetical scenario, which would require frequent evaluations throughout the year to demonstrate, the study by Medina-Flores et al. (2014a) agrees to the results of this work. Medina-Flores et al. (2014a) frozen with liquid nitrogen honeycombs of bees colonies in Zacatecas and evaluated their hygienic behavior, finding no statistical correlations between dead breeding and number of honeycombs with brood.
Within the climatic factors, relative humidity did not have significant correlation with hygienic behavior, but a positive and significant correlation was found between environmental temperature and dead brood removal, indicating that as the temperature increases also increases brood removal. In a similar study carried out in Brazil at different altitudes and with Africanized bees, there was also a positive correlation between temperature and brood removal (Sousa et al., 2016). For his part, Cheruiyot et al. (2017) point out those climatic variables could exert an influence on hygienic behavior. It is possible that the temperature and the africanization enhance a greater motor activity of bees and that facilitates the dead brood removal, but this hypothesis would have to be proven in future studies, since it was not the objective of this investigation.
The hygienic behavior of bee populations studied also correlated, although negatively, with the m a.s.l, which indicates that as the apiaries were higher (in a range of m a.s.l from 1235 to 2248 m), the lower the degree of hygienic behavior of colonies. In addition, when comparing the regions, the colonies located in the plain region were significantly more hygienic than colonies of the mountain one. The above is corroborated by a lower percentage of colonies with high hygienic behavior in the mountain region (11 %), while in the plain region, the percentage of highly hygienic colonies was higher (> 34 %). These differences can probably be attributable to a higher incidence of bees with African Ancestry (Apis mellifera scutellata) in the plain area, since Africanized bees are more hygienic and have greater ability to detect, deotoper and remove sick or infested with V. Destructor that bees of European races (Pereira et al., 2013; Medina-Flores et al., 2014b Nganso et al., 2017).
It is demonstrated that the degree of Africanization of bee’s populations in Mexico shows a continuous gradient associated with m a.s.l. At lower altitudes, the degree of Africanization of colonies is greater than in mountain areas (Medina-Flores et al., 2015; Domíngues-Ayala et al., 2016; Guzmán-Novoa et al., 2020). Most colonies Africanized in Mexico manifest lower levels of infestation by V. Destructor and viral diseases and from breeding. It was due to the hygienic behavior of bees (Guzman-Novoa et al., 2012, 2013; Medina-Flores et al., 2014a, b, c; Hamiduzzaman et al., 2015), although sometimes, despite its high hygienic behavior, a combination of parasitosis such as varroosis and chalkbrood can make colonies of African bees (Medina and Vicario-Mejía, 1999). An additional evidence that supports the presumption of greater degree of Africanization of the colonies in the plain region, are the results of the Esquivel et al. (2015), who showed that the bees of colonies of the plain region were more African and defensive than the bees of colonies from the mountain region.
The fact of having found that more than 25 % of colonies evaluated had> 80 % hygienic behavior and that more than 11 % of them showed degrees of hygienic behavior> 95 % suggests that these breeding removal percentages are acceptable to start selective breeding projects of highly hygienic bees to improve colonies´ health. These percentages are greater than those reported in other works such as held by Medina-Flores et al. (2014c)in Zacatecas state, where the average brood removal at 48 h was 75 % in the most hygienic colonies. Masaquiza et al. (2017) studying colonies of africanized bees in Ecuador, found percentages of 76 % brood removal using the needle puncture method, method that envelope the hygienic behavior of bees (Espinosa-Montaño et al., 2008). Therefore, it can be concluded that the results found in this work are important because the potential reproduction of bees genotypes with high hygienic behavior, contributes to increase the resistance of colonies to diseases (Guzman-Novoa and Morfin, 2019). It is useful to reduce the use of antibiotics in the prevention and treatment of outbreaks of breeding diseases; likewise, the hygienic colonies would have a fewer mites of V. destructor than those not selected (Spivak, 1996) and could be able to stay healthy despite the existence of colonies infested in the same apiary (Russo et al., 2020). Additionally to resistance to parasitic diseases and mites, the hygienic behavior of bees has also been related to a positive effect on the production of honey (Wielewski et al., 2012; Medina-Flores et al., 2014c).
Several studies have shown that the hygienic behavior of bees is strongly influenced by genetic effects and at least seven loci control it (Arechavaleta-Velasco et al., 2011). The heritability index for this feature has been estimated between 0.17 and 0.63 (Boecking et al., 2000; Pernal et al., 2012), so it is viable to develop lugs of hygienic bees in genetic improvement programs. In fact, Spivak (1996) produced two lines of bees high and under hygienic behavior. In addition, hygienic behavior is mainly inherited by maternal route (Unger and Guzman-Novoa, 2010), so genetic progress would be expected in the progeny of future generations even when the hygienic queens are fertilized by drone of non- hygienic populations (Ivernizzi et al., 2011). All of the above allows inferring that, if selective breeding programs were initiated with colonies in regions studied, generation genetic improvement would occur for the benefit of beekeepers. The selection of colonies in the mountain region would use European bee base, while the selection of colonies in the plain region would with bees with a greater degree of africanization. To be implemented these programs, it should also be considered that Africanized bees produce less honey and are more defensive than the European ones (Uribe-Rubio et al., 2003; Guzmán-Novoa and Uribe-Rubio, 2004).
CONCLUSION
The degree of hygienic behavior of bees populations studied varies in relation to climatic and environmental conditions. Colonies established in warmer regions and at lower altitude exhibited higher degrees of hygienic behavior compared to colonies established in cooler areas and at higher altitude. It was also found that more than 25% of colonies evaluated had a high degree of hygienic behavior (> 80 %), which would initiate selective breeding projects of highly hygienic bees to improve the health of colonies, which would benefit the Apicultural industry.
ACKNOWLEDGMENTS
Thanks to the Association of Beekeepers and Pollinators from Jalisco for their support by facilitating, the use of the colonies and apiaries studied in this work.
To all collaborators in the collection of samples.
REFERENCES
Arechavaleta-Velasco ME, Hunt GJ, Spivak M, Camacho-Rea C. 2011. Loci de rasgos binarios que influyen en la expresión del comportamiento higiénico de las abejas melíferas. Revista Mexicana de Ciencias Pecuarias 2 (3): 238-298. ISSN: 2007-1124. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-11242011000300004 [ Links ]
Boecking O, Bienefeld K, Drescher W. 2000. Heritability of the Varroa-specific hygienic behavior in honey bees (Hymenoptera: Apidae). Journal of Animal Breeding and Genetics. 117 (6): 417-424. ISSN: 1439-0388. https://doi.org/10.1046/j.1439- 0388.2000.00271.x [ Links ]
Cheruiyot S, Lattorff H, Kahuthia-Gathu R, Mbugi J, Muli E. 2018. Varroa-specific hygienic behavior of Apis mellifera scutellata in Kenya. Apidologie, 49(4): 439-449. https://doi.org/10.1007/s13592-018-0570-6 [ Links ]
Contreras-Escareño F, Pérez AB, Echazarreta CM, Cavazos AJ, Macías-Macías JO, Tapia-González JM. 2013. Características y situación actual de la apicultura en las regiones Sur y Sureste de Jalisco, México. Revista Mexicana de Ciencias Pecuarias. 4(3): 387-398. ISSN: 2007-1124. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-11242013000300009 [ Links ]
Delaplane KS, van der Steen J, Guzman-Novoa E. 2013. Standard methods for estimating strength parameters of Apis mellifera colonies. Journal of Apicultural Research. 52: 1-12. ISSN: 0021-8839. https://www.tandfonline.com/doi/pdf/10.3896/IBRA.1.52.1.03 [ Links ]
Domínguez-Ayala R, Moo-Valle H, May-Itzá WJ, Medina-Peralta S, Quezada-Euán JJG. 2016. Stock composition of northern Neotropical honey bees: mitotype and morphotype diversity in Mexico (Hymenoptera: Apidae). Apidologie. 47: 642-652. ISSN: 0044-8435. https://doi.org/10.1007/s13592-015-0414-6 [ Links ]
Emsen B, Petukhova T, Guzman-Novoa E. 2012. Factors limiting the growth of Varroa destructor populations in selected honey bee (Apis mellifera) colonies. Journal of Animal and Veterinary Advances. 11: 4519-4525. ISSN:1680-5593. https://www.researchgate.net/publication/287716219_Factors_Limiting_the_Growth_of_ Varroa_destructor_Populations_in_Selected_Honey_Bee_Apis_mellifera_L_Colonies [ Links ]
Espinosa-Montaño LG, Guzman-Novoa E, Sánchez-Albarrán A, Montaldo HH, Correa-Benítez A. 2008. Estudio comparativo de tres pruebas para evaluar el comportamiento higiénico en colonias de abejas (Apis mellifera L.). Veterinaria. México39: 39-54. ISSN: 0301-5092. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0301-50922008000100004 [ Links ]
Esquivel R, Macías-Macías J, Tapia-González J, Contreras-Escareño F, de León Mantecón M, Silva-Contreras A. 2015. Selección de abejas (Apis mellifera L) con baja defensividad y su relación con el ambiente en Jalisco, México. Abanico Veterinario. 5(1): 44-50. ISSN: 2448-6132. http://www.scielo.org.mx/scielo.php?pid=S2448- 61322015000100044&script=sci_arttext [ Links ]
Gobierno del Estado de Jalisco. 2019. Municipios de Jalisco. https://www.jalisco.gob.mx/jalisco/municipios [ Links ]
Guzman-Novoa E, Uribe-Rubio JL. 2004. Honey production by European, Africanized and hybrid honey bee (Apis mellifera) colonies in Mexico. American Bee Journal. 144: 318-320. ISSN: 0002-7626. https://dx.doi.org/10.1016/B978-0-444-64046-8.00254-8 [ Links ]
Guzman-Novoa E, Hamiduzzaman MM, Espinosa-Montaño L, Correa-Benítez A, Anguiano-Baez R, Ponce-Vázquez R. 2012. First detection of four viruses in honey bee (Apis mellifera) workers with and without deformed wings and Varroa destructor in Mexico. Journal of Apicultural Research. 51: 342-346. ISSN: 0021-8839. https://doi.org/10.3896/IBRA.1.51.4.08 [ Links ]
Guzman-Novoa E, Hamiduzzaman MM, Correa-Benítez A, Espinosa-Montaño LG, Uribe-Rubio JL. 2013. A scientific note on the first detection of black queen cell virus in honey bees (Apis mellifera) in Mexico. Apidologie. 4: 382-384. ISSN: 0044-8435. https://doi.org/10.1007/s13592-012-0191-4 [ Links ]
Guzman-Novoa E, Morfin N. 2019. Disease resistance in honey bees (Apis mellifera L.) at the colony and individual levels. In: Moo-Young, M. (Ed.), Comprehensive Biotechnology. Vol. 4 (67). Elsevier: Pergamon. Pp. 811-817. ISBN-10: 0444533524. https://doi.org/10.1016/B978-0-444-64046-8.00254-8 [ Links ]
Guzman-Novoa E., Morfin N, De la Mora A, Macías-Macías JO, Tapia-González JM, Contreras-Escareño F, Medina-Flores CA, Correa-Benítez A, Quezada-Euán JJG. 2020. The process and outcome of the africanization of honey bees in Mexico: lessons and future directions. Frontiers in Ecology and Evolution. 8: 608091. ISSN: 2296-701X. https://doi.org/10.3389/fevo.2020.608091 [ Links ]
Hanusz Z y Tarasińska J. 2015. Normalization of the Kolmogorov-Smirnov and Shapiro-Wilk tests of normality. Biometrical Letters. 52(2): 85-93. https://doi.org/10.1515/bile-2015-0008 [ Links ]
Hamiduzzaman MM, Guzman-Novoa E, Goodwin PH, Reyes-Quintana M, Koleoglu G, Correa-Benítez A, Petukhova T. 2015. Differential responses of Africanized and European honey bees (Apis mellifera) to viral replication following mechanical transmission or Varroa destructor parasitism. Journal of Invertebrate Pathology. 126: 12-20. ISSN: 0022- 2011. https://doi.org/10.1016/j.jip.2014.12.004 [ Links ]
Ivernizzi C, Rivas F, Bettucci L. 2011. Resistance to chalkbrood disease in Apis mellifera L. (Hymenoptera: Apidae) colonies with different hygienic behavior. Neotropical Entomology. 40: 28-34. ISSN: 1519-566X. https://doi.org/10.1590/S1519- 566X2011000100004 [ Links ]
Magaña-Magaña M, Tavera-Cortés M, Salazar-Barrientos L, Sanginés-García J. 2016. Productividad de la apicultura en México y su impacto sobre la rentabilidad. Revista Mexicana Ciencias Agrícolas 7: 1103-1115. ISSN: 2007-0934. https://doi.org/10.29312/remexca.v7i5.235 [ Links ]
Masaquiza D, Curbelo-Rodrígues LM, Díaz-Monroy BL, Pilataxi R, Andrade-Yucailla V. 2017. Comportamiento higiénico y nivel de infestación con Varroa destructor de Apis mellifera en la zona centro del Ecuador. Revista Ecuatoriana de Investigaciones Agropecuarias. 2(1):25-30. ISSN: 2528-8172. https://doi.org/10.31164/reiagro.v2n1.5 [ Links ]
Medina LM, Vicario-Mejía E. 1999. The presence of Varroa jacobsoni mite and Ascosphaera apis fungi in collapsing and normal honey bee (Apis mellifera L.) colonies in Yucatan, Mexico. American Bee Journal. 139(10): 794-796. ISSN: 0002-7626. http://www.scopus.com/inward/record.url?eid=2-s2.0-0033247087&partnerID=MN8TOARS [ Links ]
Medina-Flores CA, Guzman-Novoa E, Aréchiga C, Gutiérrez-Bañuelos H, Aguilera- Soto JI. 2014a. Honey production and Varroa destructor infestation of Africanized honey bee (Apis mellifera) colonies with high and low hygienic behavior. Revista Mexicana de Ciencias Pecuarias 5(2): 157-170. ISSN: 2007-1124. https://doi.org/10.22319/rmcp.v5i2.3222 [ Links ]
Medina-Flores CA, Guzman-Novoa E, Hamiduzzaman MM, Aréchiga-Flores C, López-Carlos M. 2014b. Africanized honey bees (Apis mellifera) have low infestation levels of the mite Varroa destructor in different ecological regions in Mexico. Genetics and Molecular Research. 13: 7282-7293. ISSN: 1676-5680. https://doi.org/10.4238/2014.February.21.10 [ Links ]
Medina-Flores CA, Guzman-Novoa E, Espinosa-Montaño LG, Uribe-Rubio JL, Gutierrez-Luna R, Gutierrez-Piña F. 2014c. Frequency of varroosis and nosemosis in honey bee (Apis mellifera) colonies in the state of Zacatecas, Mexico. Revista Chapingo Serie Ciencias Forestales y del Ambiente. 20: 159-167. ISSNe: 2007-4018. https://doi.org/10.5154/r.rchscfa.2013.08.028 [ Links ]
Medina-Flores CA, Guzman-Novoa E, Hamiduzzaman MM, Aguilera-Soto J, López- Carlos MA. 2015. Africanization of honey bees (Apis mellifera) in three climatic regions of northern Mexico. Veterinaria México. 2(4): 1-9. ISSN: 0301-5092. https://doi.org/10.21753/vmoa.2.4.353 [ Links ]
Medina-Flores CA, Guzmán-Novoa E, Aguilera JI, López MA, Medina-Cuéllar SE. 2019. Condiciones poblacionales y alimenticias de colonias de abejas melíferas (Apis mellifera) en tres regiones del altiplano semiárido de México. Rev. mex. de cienc. Pecuarias. 10(1): 199-211. https://doi.org/10.22319/rmcp.v10i1.4387 [ Links ]
Nganso BT, Fombong AT, Yusuf A A, Pirk CWW, Stuhl C, Torto B. 2017. Hygienic and grooming behaviors in African and European honeybees-New damage categories in Varroa destructor. PLoS ONE. 12(6): e0179329. ISSN:1932-6203. https://doi.org/10.1371/journal.pone.0179329 [ Links ]
Pereira RA, Morais MM, Francoy TM, Gonçalves LS. 2013. Hygienic behavior of Africanized honey bees Apis mellifera directed towards brood in old and new combs during diurnal and nocturnal periods. Insects. 4(4): 521-532. ISSN: 2075-4450. https://doi.org/10.3390/insects4040521 [ Links ]
Pernal SF, Sewalem A, Melathopoulos AP. 2012. Breeding for hygienic behavior in honeybees (Apis mellifera) using free-mated nucleus colonies. Apidologie. 43: 403-416. ISSN: 0044-8435. https://doi.org/10.1007/s13592-011-0105-x [ Links ]
Plan Regional de Desarrollo. 2012. Región 06 Sur de Jalisco. Comité Técnico de Planeación y Evaluación de Jalisco. Gobierno del Estado de Jalisco. Pp. 211. https://transparenciafiscal.jalisco.gob.mx/sites/default/files/plan_de_desarrollo_region_0 6_sur_vp1.pdf [ Links ]
Quezada-Lucio N. 2017. Estadística con SPSS 24. Macro., ISBN: 978-612-304-548- 7. [ Links ]
Russo RM, Liendo MC, Landi L, Pietronave H, Merke J, Fain H, et al. 2020. Grooming behavior in naturally Varroa-resistant Apis mellifera colonies from North-Central Argentina. Frontiers in Ecology and Evolution. 8: 590281. ISSN: 2296-701X. https://doi.org/10.3389/fevo.2020.590281 [ Links ]
Soto-Muciño L, Elizarraras-Baena R, Soto-Muciño I. 2017. Situación apícola en México y perspectiva de la producción de miel en el Estado de Veracruz. Revista de Estrategias del Desarrollo Empresarial. 3(7): 40-64. ISSN:2444-4952. http://www.ecorfan.org/spain/researchjournals/Estrategias_del_Desarrollo_Empresarial/ vol3num7/Revista_de_Estrategias_del_Desarrollo_Empresarial_V3_N7_5.pdf [ Links ]
Sousa ARS, Araújo ED, Gramacho KP, Nunes LA. 2016. Bee’s morphometrics and behavior in response to seasonal effects from ecoregions. Genetics and Molecular Research. 15(2): gmr.15027597. ISSN: 1676-5680. https://doi.org/10.4238/gmr.15027597 [ Links ]
Spivak M.1996. Honey bee hygienic behavior and defense against Varroa jacobsoni. Apidologie. 27: 245-260. ISSN: 0044-8435. https://doi.org/10.1051/apido:19960407 [ Links ]
Tapia-González J, Alcazar-Oceguera G, Macías-Macías J, Contreras-Escareño F, Tapia-Rivera J, Chavoya-Moreno F, Martínez-González J. 2017. Nosemosis en abejas melíferas y su relación con factores ambientales en Jalisco, México. Revista Mexicana de Ciencias Pecuarias 8 (3): 325-330. ISSN: 2007-1124. http://dx.doi.org/10.22319/rmcp.v8i3.4510 [ Links ]
Tapia-González J, Alcazar-Oceguera G, Macías-Macías J, Contreras-Escareño F, Tapia-Rivera J, Petukhova T, Guzmán-Novoa E. 2019. Varroosis en abejas melíferas en diferentes condiciones ambientales y regionales de Jalisco, México. Ecosistemas y Recursos Agropecuarios. 6(17): 243-251. ISSN: 2007-9028. https://doi.org/10.19136/era.a6n17.2018 [ Links ]
Tapia-González J, Alcazar-Oceguera G, Macías-Macías J, Contreras-Escareño F, Tapia-Rivera J, Petukhova T, Guzmán-Novoa E. 2020. Ascosferosis en abejas melíferas y su relación con factores ambientales en Jalisco, México. Revista Mexicana de Ciencias Pecuarias 11(2): 468-478. ISSN: 2007-1124. https://doi.org/10.22319/rmcp.v11i2.4926 [ Links ]
Unger P, Guzman-Novoa E. 2010. Maternal effects on the hygienic behavior of Russian x Ontario hybrid honeybees (Apis mellifera L.). Journal of Heredity. 101(1) :91-96. ISSN: 0022-1503. https://doi.org/10.1093/jhered/esp092 [ Links ]
Uribe-Rubio JL, Guzmán-Novoa E, Hunt GJ, Correa-Benítez A, Zozaya RJA. 2003. The effect of africanization and honey production, defensive behavior and size of honeybees (Apis mellifera L.) in the Mexican high plateau. Veterinaria México. 34: 47-59. ISSN: 0301-5092. https://www.medigraphic.com/pdfs/vetmex/vm-2003/vm031e.pdf [ Links ]
Wagoner KM, Spivak M, Rueppell O. 2018. Brood affects hygienic behavior in the honey bee (Hymenoptera: Apidae). Journal of Economic Entomology. 111: 2520-2530. ISSN: 0022-0493. https://doi.org/10.1093/jee/toy266 [ Links ]
Wielewski P, de Toledo VAA, Nunes ME, Costa-Maia FM, Faquinello P, Lourenco DAL, Ruvolo-Takasusuki MCC, Oliveira, CAL, Sereia MJ. 2012. Relationship between hygienic behavior and Varroa destructor mites in colonies producing honey or royal jelly. Sociobiology. 59(1): 251-274. ISSN: 0361-6525. http://dx.doi.org/10.13102/sociobiology.v59i1.682 [ Links ]
William H, Ahrens, Darrell J, Girish Budhwar. 1990. Use of the Arcsine and Square Root Transformations for Subjectively Determined Percentage Data. Weed Science, 38(4): 452-458. http://www.jstor.org/stable/4044902 [ Links ]
Xonis C, Thrasyvoulou A, El Taj HF. 2015. Variability of hygienic behavior in bee Apis mellifera macedonica. Bulgarian Journal of Agricultural Science. 21(3): 680-685. ISSN: 1310-0351. https://www.agrojournal.org/21/03-30.pdf [ Links ]
Received: December 08, 2020; Accepted: March 31, 2021











 texto em
texto em