Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Abanico veterinario
On-line version ISSN 2448-6132Print version ISSN 2007-428X
Abanico vet vol.10 Tepic Jan./Dec. 2020 Epub Mar 02, 2021
https://doi.org/10.21929/abavet2020.8
Short Note
Exploratory study of the genotoxicity from recombinant bovine tuberculosis vaccines
1Laboratorio de Toxicología Genética. Departamento, Salud pública, División Ciencias Veterinarias, Centro Universitario de Ciencias Biológicas y Agropecuarias, Universidad de Guadalajara. México.
2Laboratorio de Caracterización Molecular de Patógenos. Departamento de Salud Pública, División Ciencias Veterinarias, Centro Universitario de Ciencias Biológicas y Agropecuarias, Universidad de Guadalajara. México.
3Biotecnología Médica y Farmacéutica Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, A.C. México.
4Laboratorio de Hidrobiología y Ecotoxicología Acuática, Dirección Departamental de Biotecnología y Ambientales, Universidad Autónoma de Guadalajara. México.
5Laboratorio de Evaluación de Genotóxicos, Programa Internacional de Medicina, Universidad Autónoma de Guadalajara. México.
The BCG (Bacillus Calmette-Guérin) vaccine for the control of bovine tuberculosis has variable efficacy and the generation and testing of new vaccines is required. For this reason, an exploratory study was carried out to evaluate the genotoxicity of two potential vaccines, recombinant bovine tuberculosis in Holstein Freisan calves with an average age of 9 months, using micronucleated erythrocytes (MNE). Five groups were formed: 1) Saline solution, 2) The vector pVAX1 (Vector without insert), CV), 3) Vaccine Mycobacterium bovis (M. bovis) type 1 (PE11 [VR1]), 4) Vaccine M. bovis type 2 (PPE68 [VR2]), 5) Both vaccines (VR1 + VR2). Five blood samples were taken from each organism: the first one prior to treatment, the second to the fourth sample every 24 hours and the fifth one 90 days after treatment. The samples were analyzed with microscopy and MNE/10,000 erythrocytes were counted. MNE frequencies decreased with age (Kruskall Wallis, 95%). When analyzing the treatments with respect to the control, a lower value of MNE was identified in the VR2 groups and in VR1 + VR2 (P = 0.02). These results appear to have a cytoprotective effect; however, it could be a masked myelosuppressive (cytotoxic) effect, since the frequency of MNE decreases due to myelosuppression. To confirm cytotoxicity, it is to continue the study in younger organisms suggested.
Keywords: Genotoxicity; micronuclei; cattle and recombinant antituberculous vaccine
La vacuna BCG (bacilo Calmette-Guérin) para el control de la tuberculosis bovina tiene eficacia variable y se requiere la generación y prueba de nuevas vacunas. Por ello se realizó un estudio exploratorio para evaluar la genotoxicidad de dos potenciales vacunas, recombinantes antituberculosa bovina en becerras Holstein Freisan de edad promedio de 9 meses, mediante eritrocitos micronucleados (EMN). Se formaron 5 grupos: 1) Solución salina, 2) El vector pVAX1 (Vector sin inserto), CV), 3) Vacuna Micobacterium bovis (M. bovis) tipo 1 (PE11 [VR1]), 4) Vacuna M. bovis tipo 2 (PPE68 [VR2]), 5) Ambas vacunas (VR1+VR2). A cada organismo se le tomaron 5 muestras de sangre: la primera previa al tratamiento, de la segunda a la cuarta muestra cada 24 horas y la 5ta a los 90 días postratamiento. Las muestras se analizaron con microscopía y se contabilizaron EMN/10,000 eritrocitos. Frecuencias de EMN disminuyeron con la edad (Kruskall Wallis, 95%). Al analizar los tratamientos con respecto al control se identificó menor valor de EMN en los grupos VR2 y en VR1+VR2 (P=0.02). Estos resultados, aparentan efecto citoprotector, no obstante, podría tratarse de efecto mielosupresor (citotóxico) enmascarado, ya que la frecuencia de EMN disminuye al haber mielodepresión. Para confirmar citotoxicidad se sugiere continuar el estudio en organismos más jóvenes.
Palabras clave: Genotoxicidad; micronúcleos; bovinos y vacuna recombinante antituberculosa
INTRODUCTION
The World Health Organization indicates that bovine tuberculosis is a chronic, zoonotic bacterial infectious-infectious disease of high morbidity and mortality and is endemic to developing countries; specifically in Mexico, it is prevalent throughout the country (OIE, 2020; PRONABIVE, 2015), where natural transmission in livestock is likely to be favored (Van der Heijden et al., 2017). The disease produces great economic losses due to the decrease in the population of cattle and milk; therefore, it requires constant control and prevention (Flores, 2012; Gooding y Brook, 2014; Ortíz, 2015; OIE, 2020). Mycobacterium bovis (M. bovis) and M. tuberculosis are the cause of tuberculosis. This disease forms nodules or tubers in the lymph nodes and various tissues, from where its name arises (Carriosa et al., 2015; Martínez et al., 2019).
The clinical signs can be subacute or chronic; the progressive rate is variable, in some animals the bacteria remain dormant or take years to manifest; while in others they can be seriously affected in a short time, and that is why it must be notified according to the Terrestrial Animal Health Code (OIE, 2020). It is by contact transmitted with infected individuals or tissues, by ingestion of contaminated food or body fluids (OIE, 2020; Herrera et al., 2008; Higareda-de Sales et al., 2015; Himsworth et al., 2010; Grange, 2001).
A proposal to eradicate the disease is vaccination, whose objective is to improve the immune response against tuberculosis, reduce the incidence of active disease; in addition to more durable protection, greater efficacy and safety, applicability to any population and compatibility with the rest of the vaccination schedule; as well as low cost (Barba et al., 2013).
For its part, the micronucleus (MN) test detects the loss of fragments or complete chromosomes during mitosis, and in peripheral blood. It is an excellent inexpensive, highly sensitive and informative tool does not require large sample sizes to assess possible damage to the DNA. Furthermore, in vivo models have the characteristic of transforming substances where it has been described that many of their metabolites can become more toxic than the original compound; as well as the immune response can be evaluated through activation of the bone marrow or myelosuppression (Cristaldi, 2004; Cedano etal., 2012; Torres-Bugarín et al., 2015; Castañeda et al., 2016).
For more than 100 years, the BCG vaccine (Calmette-Guérin bacillus), derived from an attenuated strain of M. bovis, has been used; called the Calmette-Guérin bacillus, for the prevention of bovine tuberculosis; this vaccine has variable efficacy (Cordero et al., 2013). Therefore, it is a priority to work on the generation of new vaccines, based on new knowledge about the genome of M. tuberculosis and M. bovis, together with their immune response (Cordero et al., 2013; Van der Heijden et al., 2017).
New strategies in the generation of vaccines, such as those of the DNA type, are tools that can be, for the prevention of bovine tuberculosis evaluated and exploited. In this regard, bovines vaccinated with a recombinant DNA variant (rDNA), which codes for the 85B, MPT64, and MPT83 antigens, have been to improve the immune response and decrease antigen loading, reported. Therefore, Barba, et al., (2013) evaluated the effectiveness of rDNA vaccines using other types of M. bovis antigens; for this, they cloned the genes that encode the proteins PE11 and PPE68, in the eukaryotic expression vector pVAX1. To do this they constructed the DNA vaccine, based on a eukaryotic expression vector (pVAX1, Invitrogen, USA), from which they produced PE11 and PPE68 proteins encoded in a gene present in RD1, a region absent from M. bovis BCG, a strain commonly used as a vaccine in humans and in experimental bovine models.
This strain contributes to cross reactivity and confusion between vaccinated and infected animals, when tested. They used pVAX1-PPE68 and PE11 to vaccinate Holstein Cattle, and to determine their ability to induce IFN-g production in vitro, as well as to generate antibodies in vaccinated animals.
RD1 is a 9.5 kb section of DNA called deletion region 1, it is present in virulent M. tuberculosis strains, but is removed in all attenuated strains of the M. bovis BCG vaccine. This region encodes at least nine genes. Some or all of the RD1 gene products may be involved in virulence and pathogenesis (Daugelat, et al., 2003).
Although the construction of new vaccines is necessary, it is also essential that these new treatments go through an evaluation process, both of their therapeutic efficacy, as well as possible toxic or genotoxic effects, in the short or long term.
Therefore, the objective of the present study was to evaluate the genotoxicity of recombinant tuberculosis vaccines developed by Barba, et al., (2013) in Holstein Friesian calves (Bos taurus).
MATERIAL AND METHODS
Type of study
Experimental, observational, longitudinal, comparative. With registration number CINV.020/15 before the Research Coordination of the University Center for Biological and Agricultural Sciences of the University of Guadalajara, Jalisco, Mexico.
Organisms
We worked with 13 healthy Holstein Friesian (Bos taurus) calves, with an average age of 9 months, from the Production Cofradía ranch, University of Guadalajara, Jalisco, Mexico. In biomedical research, working with animals of rare use represents difficulties, such as the ideal size and number of animals, as well as maintenance and care. As the OECD points out, bovines are a kind of rare use in biomedical research; due to ethics, methodology and high cost, the principle of using the minimum number of animals must be respected. In the particular case where the study was carried out, it was not possible to use the ideal number of calves, due to the logistics of care, accommodation and feeding (OCDE, 1999).
Groups of study
Five groups were formed, to which a 1.0 mL dose was administered intramuscularly, of one of the following compounds:
Group 1) [n=3] Control Sterile saline solution at 0.9% (p/v) (CSS). Group 2) [n=2] Vector Control without insert pVAX1 (CV).
Group 3) [n=3] Recombinant vaccine encoded for PE11: 0.5 mg/mL plasmid M. bovis (VR1).
Group 4) [n=3] Recombinant vaccine encoded for PPE68: 0.5 mg/mL plasmid M. bovis (VR2).
Group 5) (n = 2] VR1+VR2 (PE11: 0.25 mg/mL+PPE68: 0.25 mg/mL).
Characteristics of recombinant vaccines
-
Vector pVAX1 (Invitrogen Thermo Fisher Scientific Cat. No. V26020)
This vector was designed according to FDA guidelines; eukaryotic DNA sequences are limited to those required for expression in order to minimize the possibility of chromosomal integration (Barba et al., 2013).
-
Preparation of PE11 and PPE68 vaccines
These were prepared at the Jalisco State Center for Research and Assistance in Technology and Design (CIATEJ), where the coding regions of the PE11 and PPE68 genes were amplified from genomic DNA from M. bovis; by polymerase chain reaction with oligonucleotides, specific and expressly designed for this project. The identity and fidelity of the amplified sequence was verified by restriction with type II endonucleases (XbaI and HindIII) and sequencing. These were then inserted into the eukaryotic expression vector pVAX1. Plasmid DNA was purified using a commercial Quiagen Plasmid Plus Midi kit (Barba et al., 2013).
-
PE11 and PPE68 proteins
These families of globular structure proteins are those that confer the greatest variability to M. tuberculosis. The PE11 protein (Proline-Glutamic Acid motifs at the level of the N- terminus), are characterized by being rich in proline and glutamic acid, and the PPE68 protein (proline, proline, glutamic acid) is defined by tandem repeats (MPTRs) (Fontalvo Rivera y Gómez Camargo, 2015).
-
Generation of recombinant vaccines
Recombinant plasmids transformed into Escherichia coli DH5α, and containing the PE11 or PPE68 proteins, were obtained by means of the commercial Quiagen Plasmid Plus Maxi kit, and mixed with isotonic saline solution (SSI); until adjusted to a volume of 0.5 mL, with a concentration of 500 µg/mL (administered vaccine). The PE11 and PPE68 genes were amplified by PCR with the help of the high-fidelity Phusion enzyme (Finnizymes, USA) and the M. bovis AN5 genomic DNA as template, and the primer pair MbPPE68-5FH3 (5´-GGAGAAGCTTGTCACCATGCTGTGG- 3)+MbPPE683RXb3RXb(5´-GATCCGCTCTAGATTACCTGCCTCCTG-3').
The PCR products were digested with HindIII and XbaI (New England Biolabs, USA), and then ligated with the pVAX1 vector, using the same restriction sites. Cloning was confirmed by restriction digestion, and the ratio between identity and fidelity of the inserted gene. Then verified by sequencing performed at the Genomics Laboratory for Biodiversity (LANGEBIO, Cinvestav Irapuato, Mexico). The expression of cloned genes in pVAX1 depends on its CMV promoter, as well as on the Kozak and ATG sequences that are incorporated into the primers given its absence in pVAX1; as pointed out by Barba and his collaborators (Barba et al., 2013).
Sample collection and processing
Peripheral blood samples were taken from each calf, the first one prior to treatment; from the 2nd to the 4th every 24 hours, and the last after 90 days. Needle puncture number 18 took the sample from the jugular, and two smears were made per sample, which were left to dry in the open air and fixed in 96% ethanol for 10 minutes. The second smear was for backup purposes. Subsequently, they were stained with acridine orange, specific staining for nucleic acids (Hayashi, 1990) and they were kept until their analysis in boxes to keep them free of dust and exposure to light (Torres-Bugarín et al., 2015).
Sample analysis
The technician responsible for the analysis of the samples was unaware of the information related to them, who per sample counted 10,000 total erythrocytes (ET), to identify the values of micronucleated erythrocytes (MNE), through a microscope equipped with fluorescence (Zeiss® brand) under 100X objective. It was considered as MNE when it presents in its cytoplasm a small, round or oval structure, well defined with a bright yellow color (characteristic tone of DNA due to acridine orange staining, which is specific for nucleic acids). When focusing and blur the objective, it is in the same plane of the cell as seen in figure 1 (Torres-Bugarín et al., 2015).
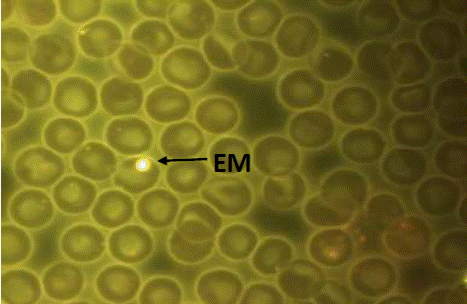
Figure 1 Peripheral blood smear. Micronucleated normochromatic erythrocyte (MNE). Acridine orange stain. Carl Zeiss Binocular Microscope Mod. Axioscope A1® IVFL Fluorescence Filter 450 to 490 nanometers, Axiocam MRc3 Rev1 Camera, 100x/1.25 Planochromatic Objective. Image captured at 1,000 actual magnifications.
Statistical analysis
For the comparison of the MNE values, previous evaluations of bias, kurtosis and homoscedasticity indices were made, to discriminate between the use of ANOVA or Kruskal Wallis and proceed to their analysis. The value of P <0.05 was considered significant and, if appropriate, LSD tests were performed to locate homogeneous groups. The program STATGRAPHICSä Centurion, ver. 15 (StatPoint, USA).
Ethical considerations
All animals were treated in accordance with the procedures established by the Official Mexican STANDARD NOM-062-ZOO-1999, technical specifications for the production, care and use of laboratory animals (NOM-062-ZOO-1999; OCDE, 1999).
RESULTS
Table 1 shows the sample size, the individual and mean values, and the standard errors of the baseline values of the MNE. It should be noted that VR2 and VR1+VR2, presented a frequency of MNE s with a dose response effect (P=0.02, Kruskal Wallis).
Table 1 Values of micronucleated erythrocytes (MNEs) in peripheral blood of Holstein calves exposed to recombinant test vaccines over time
| Sampling | Values of MNE /10,000 erythrocytes totals | ||||
|---|---|---|---|---|---|
| CSS, (n=3) | CV, (n=2) | VR1,(n=3) | VR2, (n=3) | VR1+VR2, (n=2) | |
| Day 1 | 2.6 ± 1.98 [n=13] | ||||
| Basal Values | |||||
| Day 2 | 2, 2, 5 | 1, 2 | 1, 3, 4 | 1, 1, 3 | 1, 2 |
| Day 3 | 1,1,3 | 1, 1 | 2, 2, 2 | 1, 1, 2 | 2, 2 |
| Day 4 | 1, 3, 4 | 1, 2 | 0, 2, 3 | 1, 1, 2 | 2, 1 |
| Day 90 | 1, 2, 3 | 1, 2 | 0, 2, 3 | 0, 1, 2 | 2, 2 |
| General Average | 2.4 ± 0.3 | 1.4 ± 0.2 | 2.5 ± 0.5 | 1.3 ± 0.2 | 2.0 ± 0.3 |
Individual values and average ± standard error are shown. MNE: micronucleated erythrocytes; CSS: control saline solution; CV: vehicle control; VR1: recombinant vaccine (PE-11); VR2: recombinant vaccine (PPE-68); VR1+VR2: recombinant vaccine (PE-11+PPE-68); n: sample size.
When analyzing the MNE values of all the organisms in the different groups and sampling days, it was identified that the data do not comply with the principle of homogeneous variances and normality. Therefore the Kruskal Wallis test was applied, with which it was found that MNE values show apparent decrease over time, but without statistical significance (P=0.70), (Figure 2).
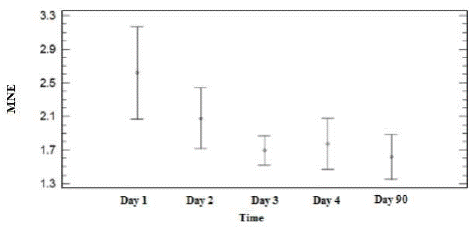
Figure 2 Values of micronucleated erythrocytes (MNE/10,000 erythrocytes) in peripheral blood of Holstein Freisan calves in the different study groups and sampled on different days. The charts show mean and standard error
In table 1 and figure 3, the dose response effect (P=0.02, Kruskal Wallis) on the MNE frequencies of the VR2 application can be observed. Furthermore, it should be noted that CSS and VR1 groups, as well as CV and VR2 groups behave in a very similar way (P>0.05); while the VR1+VR2 group presents intermediate MNE values between VR1 and VR2 (P> 0.05), see figure 3.
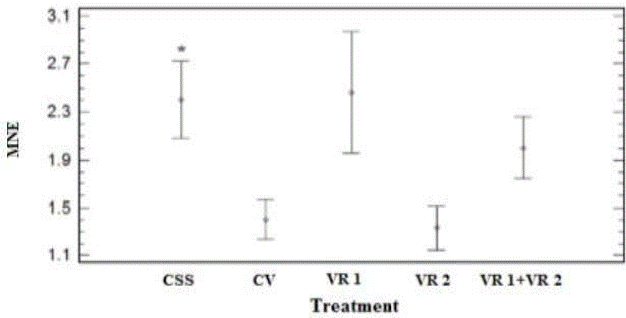
Figure 3 Values of peripheral blood micronucleated red cells (MNE/10,000 ET) from treated Holstein Freisan calves. CSS: Saline solution, CV: Vector without insert (pVAX1), VR1: Recombinant vaccine encoded for PE11 (PE11/0.5 mg/mL plasmid Micobacterium bovis (M. bovis), VR2: Recombinant vaccine encoded for PPE68 (PPE68/0.5 mg/mL of plasmid M. bovis, VR1+VR2 (PE11/0.25 mg/mL+PPE68/0.25 mg/mL). Data shows mean and standard errors. CV, VR2 and VR1+VR2 vs CSS, (P=0.02, Kruskal Wallis)*.
DISCUSSION
The Holstein Freisan vaccine breed as a bioindicator of genotoxicity
Before evaluating the genotoxicity of an agent by means of the MN test in peripheral blood in an organism that has not been as a bioindicator of genotoxic agents, tested, the spontaneous values of this species must be available (Zúñiga-González et al., 2001). In the specific situation of cattle, the spontaneous MNE/10,000 erythrocyte value described is 2.4±1.7 (mean and standard error) (Zúñiga et al., 1996); and experimental studies of genotoxic are scarce. Only one investigation was found that describes the behavior of the adult Latvian Brown breed, exposed to electromagnetic radiation, and it is noted that the MNE values were 0.6/1,000 vs. 0.1/1,000 erythrocytes, in unexposed animals (Balode, 1996). For the specific case of the Holstein Freisan breed, there was no history, but this working group found spontaneous MNE values of 2.6±1.98/10,000 erythrocytes (mean and standard error) in 9-month-old Friesian animals. Value very similar to that previously described by Zúñiga et al., (1996); however, in this last study, the sex, breed or age of the cattle studied was not specified. The MNE values found in these animals would suppose low efficiency, as a bioindicator of genotoxic agents, which could improve if working with younger calves (Zúñiga et al., 2001). These results motivated the evaluation of the spontaneous values of MNE and polychromatic erythrocytes (EPC) in Holstein Freisan calves 24-48 h old; in such a way that the peripheral blood samples were taken from newborn organisms of different breeds, as observed in Table 2. Indeed, it was confirmed that the age of the animals determine the values of MNE and EPC, and it was possible to determine that the youngest animals present the highest values, and as observed in Table 2, this pattern is repeated in calves of the Simmental and Brown Swiss breeds (Villa, et al., 2015).
This phenomenon has been observed in other species, since the spontaneous values of MNE in peripheral blood of many species, such as the rat, squirrel and the human, depend on the maturation of the spleen, and the spleen matures with age (Zúñiga-González et al., 2001; Batista-González et al., 2006).
In the present study, it can be seen that the frequency of MNE tends to decrease with age (Table 2), which agrees with what has already been described (Zúñiga-González et al; 2001). For this reason, the use of young animals is recommended, and this work is an antecedent in research with cattle; where it can be clearly seen that the age of the bovines is a factor to consider in the MNE and EPC values.
Table 2 Polychromatic erythrocytes and spontaneous micronucleates in different breeds of calves
| Races | n | Age | MNE /10,000 ET | EPC/1000 ET | Reference |
|---|---|---|---|---|---|
| 13 | 9 m | 1.6 ± 0.3 | 0 | This paper | |
| Holstein Friesian | 13 | 6 m | 2.6 ± 0.5 | 0 | This paper |
| 5 | 24-48 h | 7.4 ± 1.0 | 7.4 ± 2.3 | Villa, et al., 2015 | |
| Swiss Brown | 5 | 24-48 h | 7.8 ± 0.7 | 9.0 ± 3.9 | Villa, et al., 2015 |
| Simmental | 5 | 24-48 h | 6.4 ± 0.7 | 11.8 ± 4.3 | Villa, et al., 2015 |
n: size of sample; h: hours; m: months; EPC: Polychromatic Erythrocytes; MNE: micronucleate; average and standard error.
Genotoxic effect on the immune response of recombinant test vaccines
The objective of the Barba et al., (2013), project was to propose new recombinant vaccines for the control of bovine tuberculosis. After the design of the test vaccines, they were limited by the availability of animals, so the reduced sample sizes were decided, in order to evaluate the necessary control and experimental groups. Therefore, in the present exploratory study describing the genotoxic effect, it was not possible to adjust the optimal sample size.
When analyzing the genotoxic effect of the recombinant tuberculosis vaccines, it was identified that the MNE values were lower with a dose-response effect in the groups treated with VR2; this can be interpreted as a cytoprotective or antigenotoxic effect; however, these results should be taken with caution, since there are two points to consider:
The first of these was the sample size, which, although the MN test is highly sensitive and does not require large sample sizes. The ideal would have been to work with more than five animals per group, but due to the cost, size and age of these animals, only two or three calves per group were available; and this may have skewed the results.
The second point is that to determine genotoxicity by means of the micronucleus test in peripheral blood erythrocytes, the mitotic activity of the bone marrow must be considered, which is evaluated by the presence or absence of EPC in peripheral blood. The EPCs are young red blood cells that have a maximum of 24 hours of being released; this because the formation of MN is carried out during the anaphase-telophase stage of cell division; and if this does not occur, the MNs cannot form. However, if there is cell division in the bone marrow (myeloproliferation), the micronucleogenic or genotoxic effect of the test substances can be easily observed; in opposition; if the latter inhibits cell division (myelosuppression); that is, if there is cytotoxicity, then it will not be possible to observe the increase in MNE in peripheral blood (genotoxic effect).
Therefore, going back to figure 3, where it is observed that the values of MNE in the VR2 and VR1+VR2 groups is lower than in the CSS group (P=0.02). What may have actually happened is that the use of VR2 is significantly affecting the calf's bone marrow, to the point of having induced myelosuppression due to the generated immune activity. However, in this study there is no way to corroborate this effect using the classical technique, by quantifying polychromatic red blood cells in peripheral blood, since calves with an average age of 6 to 9 months do not present this type of cells (Table 2 ).
Barba et al., (2013), in parallel demonstrated that the DNA vaccine pVAX1-PPE68 (VR2) constructed and evaluated in Holstein Freisan cattle, induced the production of IFN-γ cytokines (proteins produced by immunocytes; in response to an antigen, generally viral) in animals vaccinated above background levels. In addition, it was able to induce the production of antibodies directed against proteins that cross-react in complete cell extracts of M. bovis BCG; unlike the recombinant vaccine pVAX1-PE11 (VR1), which did not induce an IFN-γ-mediated immune response, at least at levels detectable by the commercial kit used Barba et al., (2013). In other words, then, only the VR2 vaccine produced an immune response and also a change in the frequency of micronucleated cells (only the VR2 vaccine had an effect on this biomarker); even when the administered dose was half, the response was 50%, less in relation to the group treated with saline solution (CSS), as observed in figure 3. This indicates that VR2 produces an effect on bone marrow, to such an extent that it affects the formation of MNEs. From this, it follows that the most likely immunological activity induced by the VR2 vaccine also causes myelosuppression.
This study, although preliminary, in addition to having shown a dose-response effect of recombinant tuberculosis vaccines, it also raises a big question, can recombinant tuberculosis vaccines have myelosuppression as a side effect? It is noteworthy that the versatility of the micronucleus test in peripheral blood erythrocytes also allows evaluating the myelosuppressive effect of recombinant vaccines.
CONCLUSIONS
In this work, although preliminary, it yielded very valuable results; a dose response effect of VR2 was in the MNE formation, detected. However, the results could be masked by possible myelosuppression (cytotoxicity), an effect that was not corroborated, since bovines at this average age of 9 months do not present polychromatic erythrocytes in peripheral blood; it is suggested to work with bovines less than one month old and a larger number of animals. These findings motivate us to continue investigating the induction of genomic instability and cytotoxicity of vaccines in general, and in particular anti- tuberculosis drugs, since their role in bone marrow and in the integrity of genetic material is unknown, both in experimental organisms and in farm.
LITERATURA CITADA
Balode Z. 1996. Assessment of radio-frequency electromagnetic radiation by the micronucleus test in Bovine peripheral erythrocytes. Science of the Total Environment. 180(1): 81-85. https://doi.org/10.1016/0048-9697(95)04923-1 [ Links ]
Barba J, Flores-Valdez MA, Aceves-Sánchez MJ, Pacheco-Gallardo C, Álvarez AH, González-Aguilar D. 2013. A DNA Vaccine Containing PPE68 Induces Humoral Response in Cattle. Intern J Appl Res Vet Med. 11 (2):96-99. https://www.researchgate.net/publication/251231209_A_DNA_vaccine_containing_PPE68_induces_humoral_response_in_cattle [ Links ]
Batista GC, Corona RR, Gómez MBC, Zamora PAL, Ramos IML, Zuñiga G. 2006. Micronucleated erythrocytes in preterm newborns in relation to maternal pathology. Rev Biomed. 17: 11-16. https://www.medigraphic.com/pdfs/revbio/bio-2006/bio061c.pdf [ Links ]
Carriosa UJ, Flores VE, Gutiérrez RJA, Juárez LNO. 2015. Evaluación del grado de concordancia entre los resultados del examen histopatológico y del cultivo bacteriológico en el diagnóstico de tuberculosis bovina en México. Veterinaria México OA. 2(3): 1-12. ISSN: 2448-6760. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2448-67602015000300002 [ Links ]
Castañeda-Yslas JI, Arellano-García ME, García-Zarate MA, Balam Ruíz-Ruíz B, Zavala-Cerna MG, Torres-Bugarín O. 2016. Biomonitoring with micronuclei test in buccal cells of female farmers and children exposed to pesticides of Maneadero Agricultural Valley, Baja California, México. Journal of Toxicology. 2016:1-8. https://doi.org/10.1155/2016/7934257 [ Links ]
Cedano DA, Martínez GS, Escalera VF, Salgado MS, Carrillo DF, Macías CH, Peña PB. 2012. La prueba de micronúcleos en sangre como bioindicador de genotóxicos. Abanico Veterinario. 2(2):43-52. https://www.medigraphic.com/cgi-bin/new/resumen.cgi?IDARTICULO=44884 [ Links ]
Cordero CAM, Gil PR, Gil de MA. 2013. Actualización de las nuevas vacunas frente a la tuberculosis. Medicina respiratoria. 6(1):45-52. http://www.neumologiaysalud.es/descargas/R6/R6-6.pdf [ Links ]
Cristaldi M, Leardi LA, Udroui I, Zilli R. 2004. Comparative evaluation of background micronucleus frequencies in domestic mammals. Mutat Res. 559:1-9. https://doi.org/10.1016/j.mrgentox.2003.10.021 [ Links ]
Daugelat S, Kowall J, Mattow J, Bumann D, Winter R, Hurwitz R, Kaufmann SH. 2003.The RD1 proteins of Mycobacterium tuberculosis: expression in Mycobacterium smegmatis and biochemical characterization. Microbes Infect. 5(12):1082-95. https://doi.org/10.1016/S1286-4579(03)00205-3 [ Links ]
Flores SHO. 2012. Prevalencia y pérdidas económicas provocadas por tuberculosis bovina (Mycobacterium bovis) en una planta faenadora de la Región de Los Lagos, 2006- 2010. Boletín Veterinario Oficial. 14: 1-16 https://www2.sag.gob.cl/Pecuaria/bvo/BVO_15_I_semestre_2012/articulos_PDF/regiones/prevalencia_TB_tesis_HFlores.pdf [ Links ]
Fontalvo Rivera D, Gómez Camargo D. 2015. Genes del Mycobacterium tuberculosis involucrados en la patogenicidad y resistencia a antibióticos durante la tuberculosis pulmonar y extrapulmonar. MÉD.UIS. 28(1):39-51. http://www.scielo.org.co/pdf/muis/v28n1/v28n1a04.pdf [ Links ]
Gooding MR, Brook RK. 2014. Modeling and mitigating winter hay bale damage by elk in a low prevalence bovine tuberculosis endemic zone. Preventive Veterinary Medicine. 114(2):123-131. https://doi.org/10.1016/j.prevetmed.2014.01.005 [ Links ]
Grange JM. 2001. Mycobacterium bovis infection in human beings. Tuberculosis. 81(1-2): 71-77. https://doi.org/10.1054/tube.2000.0263 [ Links ]
Hayashi M, Morita T, Kodama Y, Sofuni T, Ishidate Jr M. 1990. The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange-coated slides. Mutation Research Letters. 245(4):245-249. https://doi.org/10.1016/0165-7992(90)90153-B [ Links ]
Herrera LE, Estrada CC, Díaz MDM, Huitron NG. Femat FAR. 2008. Oportunidades para modelar y controlar enfermedades que afecta al ganado bovino en México. https://repositorio.ipicyt.edu.mx/handle/11627/3417 [ Links ]
Higareda-de Sales LG, Ramírez CFJ, Razo IF. Milián SF, Aguilar TG, Herrera RSE. 2015. Potencial presencia de tuberculosis zoonótica en la región Altos Sur de Jalisco, México. Rev Sal Jal. 2(1):25-29. https://www.medigraphic.com/cgi-bin/new/resumen.cgi?IDARTICULO=77291 [ Links ]
Himsworth CG, Elkin BT, Nishi JS, Tasha Epp T, Lyashchenko KP, Om Surujballi O, Turcotte C, Esfandiari J, Greenwald R, Leighton FA. 2010. Comparison of test performance and evaluation of novel immunoassays for tuberculosis in a captive herd of Wood bison naturally infected with Mycobacterium bovis. Journal of Wildlife Diseases. 46(1): 78-86. https://doi.org/10.7589/0090-3558-46.1.78 [ Links ]
Martínez JC, Llerena C, Valbuena YA. Importancia de Investigar Mycobacterium bovis en muestras clínicas de procedencia humana. 2019. Biomédica, Revista del Instituto Nacional de Salud. 39(Sp 1). https://doi.org/10.7705/biomedica.v39i2.4358 [ Links ]
NOM-062-ZOO-1999, NORMA Oficial Mexicana. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. 1999. http://www.ibt.unam.mx/computo/pdfs/bioterio.NOM-062.pdf [ Links ]
OCDE (Organización para la Cooperación y el Desarrollo Económicos). Guía para el cuidado y uso de los animales de laboratorio. Edicion Mexicana auspiciada por la Academia Nacional De Medicina.1999. Copyright National Academy Press, Washington, D.C. 19962019. https://www.uss.cl/wp-content/uploads/2014/12/Gui%CC%81a-para-el-Cuidado-y-Uso-de-los-Animales-de-Laboratorio.pdf [ Links ]
OIE (Organización Mundial de Sanidad Animal, proteger a los animales, preservar nuestro futuro) 2020. Tuberculosis bovina. https://www.oie.int/es/sanidad-animal-en-el-mundo/enfermedades-de-los-animales/tuberculosis-bovina. [ Links ]
Ortiz MEP. 2015. Tuberculosis bovina: un problema aún sin resolver. CIBA Revista Iberoamericana de las Ciencias Biológicas y Agropecuarias. 4(8):162-169. https://doi.org/10.23913/ciba.v4i8.32. http://ciba.org.mx/index.php/CIBA/article/view/32 [ Links ]
PRONABIVE (Productora Nacional de Biológicos Veterinarios, Gobierno de México). 2018. Tuberculosis bovina en México. https://www.gob.mx/pronabive/articulos/tuberculosis-bovina-en-mexico [ Links ]
Torres-Bugarin O, Ramos IML, Ruíz BS, Flores GA, Zavala MG. 2015. La Prueba de micronúcleos: Biomarcador de contaminación genotóxica, mutagénica y/o teratogénica. En: Botello AV, Páez-Osuna F, Mendez-Rodriguez L, Betancourt-Lozano, M, Álvarez- Borrego S, Lara-Lara R. Ed. Pacífico Mexicano. Contaminación e impacto ambiental: diagnóstico y tendencias. UAC, UNAM-ICMYL, CIAD-Mazatlán, CIBNOR, CICESE, México. Pp. 819-848. ISBN: 978-607-7887-94-2 [ Links ]
Van Der Heijden EMDL, Chileshe, Vernooij JJCM, Gortazar C, Juste RA, Sevilla I, Crafford JE, Rutten VPMG, Miche AL. 2017. Immune response profiles of calves following vaccination with live BCG and inactivated Mycobacterium bovis vaccine candidates. PLoS ONE. 12(11):1-20. e0188448. https://doi.org/10.1371/journal.pone.0188448 [ Links ]
Villa Castellanos JS, Ramos-Ibarra ML, Barba León J, Mario Flores Valdez M, Torres-Bugarín O. 2015. Frecuencia de eritrocitos micronucleados en becerras expuestas a vacunas recombinantes y en terneros no expuestos al biológico. En Carvajal S, García Sahagún ML. Progreso en las Ciencias Biológico - Agropecuarias 2014. Editorial Universidad de Guadalajara. Pp. 129. http://www.cucba.udg.mx/sites/default/files//adjuntos/xxv_snicyt_20150503_1113.pdf [ Links ]
Zúñiga G, Torres-Bugarin O, Ramírez-Muñoz MP, Ramos A, Fanti-Rodríguez E, Portilla E, García-Martínez D, Cantú JM, Gallegos-Arreola MP, Sánchez-Corona J. 1996. Spontaneous micronuclei in peripheral blood erythrocytes from 35 mammalian species. Mutation Research/Genetic Toxicology and Enviromental Mutagenesis. 369:123-127. https://doi.org/10.1016/S0165-1218(96)90056-7 [ Links ]
Zúñiga-González G, Torres-Bugarín O, Zamora-Peréz A, Gómez-Meda BC, Ramos-Ibarra ML, Martínez-González S, González-Rodríguez A, Luna-Aguirre J, Ramos-Mora A, Ontiveros-Lira D, Gallegos-Arreola MP . 2001. Differences in the number of micronucleated erythrocytes among young and adult animals including humans. Spontaneous micronuclei in 43 species. Mutation Research. 494:161-67. https://doi.org/10.1016/S1383-5718(01)00180-2 [ Links ]
Received: January 15, 2019; Accepted: May 01, 2020











 text in
text in 


