Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Abanico veterinario
On-line version ISSN 2448-6132Print version ISSN 2007-428X
Abanico vet vol.10 Tepic Jan./Dec. 2020 Epub Mar 02, 2021
https://doi.org/10.21929/abavet2020.38
Original article
Seasonal treatment with amitraz against Varroa destructor and its effects in honey bee colonies of Apis mellifera
1Colegio de Postgraduados Campus Salinas de Hidalgo, San Luis Potosí, México.
2Unidad Académica de Medicina Veterinaria y Zootecnia, Universidad Autónoma de Zacatecas, México.
The objective of this work was to determine the amitraz treatment effect against Varroa destructor on the population and food reserves of honeybee colonies, during the four seasons of the year in Mexico's central high plateau. 48 colonies with similar sister queens, homogeneous populations, food reserves, and Varroa infestation levels were used.12 colonies received acaricidal treatment in the summer, 12 in winter, 12 in summer and winter, and 12 were untreated. The Varroa infestation levels were determined in adult bees, worker brood, and by mites on the hives' floor for one year. The adult bee population, capped brood area, honey, pollen, and colony weight were also evaluated. There were statistical differences (P<0.05) between the Varroa levels on treatments. Ending the experiment (spring) the infestation level in colonies treated in summer (602 ± 114) and not treated (416 ± 86) were higher (P = 0.0002) than those treated in summer and winter (109±50), or only in winter (100±42), between which, there were no statistical differences. However, there were no significant effects of the treatments on population bees, food stores, and weight. The winter treatment was sufficient to control Varroa infestation in colonies located in Mexico's central high plateau.
Keywords: Varroa destructor; honey bees; amitraz; bee population; food stores
Se determinó el efecto del tratamiento estacional con amitraz contra Varroa destructor sobre la población y reservas alimenticias de colonias de abejas melíferas durante las cuatro estaciones del año en el altiplano central de México. Se utilizaron 48 colonias con reinas hermanas, homogéneas en población, reservas de alimentos y niveles de Varroa. 12 colonias recibieron tratamiento en verano, 12 en invierno, 12 en verano e invierno y 12 no fueron tratadas. Los niveles de V. destructor en abejas adultas, en cría y la caída de ácaros en el piso de las colmenas se determinaron durante un año. También se evaluó la población de abejas, áreas de cría, miel, polen y peso de las colonias. Hubo diferencias en los niveles de Varroa entre tratamientos (P<0.05). Finalizando el experimento (primavera), el nivel de infestación en colonias tratadas en verano (602±114) y no tratadas (416±86) fueron superiores (P=0.0002) que en las tratadas en verano e invierno (109±50) o solo en invierno (100±42), entre las cuales no hubo diferencias. Sin embargo, no hubo efecto significativo de los tratamientos sobre la población, reservas de alimentos y peso de las colonias. El tratamiento invernal fue suficiente para controlar Varroa en colonias del altiplano central de México.
Palabras clave: Varroa destructor; abejas melíferas; amitraz; población de abejas; reservas de alimento
INTRODUCTION
The Varroa destructor mite (Acari: Varroidae) (Anderson and Trueman, 2000), the cause of varroosis, is the number one health problem for beekeeping worldwide (Nazzi and Le Conte, 2016). This is because its distribution is generalized, affects brood and adult bees, transmits and predisposes to the presence of bacterial, viral and fungal diseases (Martin et al., 2010; vanEngelsdorp and Maixner, 2010; Ryabov et al., 2017). Besides, it reduces the life span of bees (Dainat et al., 2012), the population size and the honey production of the colonies (Arechavaleta and Guzmán-Novoa, 2000; Medina-Flores et al., 2011) and it is considered one of the main factors associated with the high annual loss of colonies (Marie-Pierre et al., 2010; Guzmán-Novoa et al., 2010).
In addition to the problems already mentioned by Varroa to the colonies and the beekeeping industry, the application of the acaricides used for its control represent another problem since in general all have shown adverse effects for the bees, even more so if they are not applied in a correct way. Synthetic products (fluvalinate, flumethrin and amitraz) have been the most effective, particularly amitraz, which has been shown to be less residual and toxic to bees (Gashout et al., 2018) and have fewer resistance problems in the highland region central of Mexico (Rodríguez-Dehaibes et al., 2011). However, these acaricides can affect the development of queens and drones, the learning ability and the posture of the queen (Berry et al., 2013) and cause the development of resistance of the mite (Martínez-Puc and Medina-Medina, 2011; Kamler et al., 2016). Organic acids, such as formic, reduce memory Gashout et al., 2020), and oxalic acid affects the longevity and survival of workers and breeding (Schneider et al., 2012), while monoterpenes constituents of essential oils derived from plants, can have toxic effects and reduce humoral immunity (Boncristiani et al., 2012). The acaricidal efficiency of organic acids and plant extracts is variable and depends on the applicator and its position in the hive, humidity and ambient temperature, the size of the hive, the presence of brood, among other factors (Pietropaoli and Formato, 2018).
To control the mite populations in the colonies and at the same time reduce the use of acaricides, it is necessary to identify the opportune time to apply the treatments. This will contribute to reducing the negative effects of the aforementioned acaricides. Also, the selection pressure for resistant mites, the risks of contamination of colony products, the cost of production to beekeepers due to the unnecessary application of treatments and would avoid relax the selection pressure for mite resistance (Delaplane and Hood, 1997; 1999; Delaplane, 1998; Caron, 1999; González-Cabrera et al., 2016). However, the population development of the mite differs regionally due to the variation of the breeding period in the colonies and its effects on the population dynamics of the mite (Delaplane, 1998; Caron, 1999). Consequently, the treatment threshold must be determined in specific regions. Therefore, the objective of the present study was to determine the effect of amitraz-based treatment applied in summer, winter, and summer and winter on the levels of infestation by Varroa destructor and the population and feeding conditions of honeybee colonies under conditions of the central highlands of Mexico.
MATERIAL AND METHODS
Location. The experimental apiary was located in Jalpa, Zacatecas, Mexico, at 21° 38' N and 100° 51' W, and 1,380 meters above sea level. The study area has a low deciduous forest type vegetation and a semi-dry semi-warm climate. The average annual temperature is 21.2 °C and an average annual rainfall of 700 mm (INEGI, 2019).
Experimental colonies and application of acaricidal treatments. From a population of 200 honeybee colonies housed in Langstroth-type hives, 48 were selected with similar conditions. On average, the experimental colonies consisted of eight combs covered with bees, of which five combs contained capped brood, two combs with honey and one with pollen and an average level of V. destructor infestation in adult bees of 5.3 ± 0.36%. The colonies were established in a single apiary and their queens were replaced by sister queens of the same generation and origin.
Four experimental groups were formed, each one composed of 12 colonies of bees. The colonies of the first group received treatment against Varroa in the summer (on 10, 17, 24 and 31 July 2016). The colonies of the second group received treatment during the winter (19, 26 January, 2 and 9 February 2017), group three received double treatment, and one of them in summer and another in winter (on the same dates mentioned above) and the colonies of group four received no treatment during the entire experiment. The purpose of applying double acaricidal treatment (group: summer and winter) was to cause lower levels of infestation by Varroa than in the colonies treated only in one season of the year and to make comparisons regarding the population conditions and food reserves of the colonies. In addition, some beekeepers use this protocol which is probably unnecessary and adverse for the colonies. Generating this information allows making decisions about the number of treatments and the most convenient time of application for the control of Varroa.
Treatments against Varroa consisted of applying 10 ml of amitraz (Taktic) at 1.25% on an absorbent towel (Scott) measuring 28 x 6.5 cm on a weekly basis and for four occasions on the heads of the brood chamber frames. Lupo and Gerling (1990), Smodiš et al. (2011) and Gregorc and Planinc (2012) base the use of amitraz in this preparation on previous research, in the proven effectiveness of amitraz (Semkiw et al., 2013). Also, in the low availability of specific acaricides for bees in Mexico and the need for the experiment to guarantee a significant reduction in levels of infestation, regardless of the environmental conditions of each time of year.
Population size, food reserve and weight. The population of bees, brooding areas, honey and pollen of the colonies were calculated through the average percentage estimated by two people of the surface of each side of the honeycomb occupied by these variables. To determine the population of bees, the percentage area and the number of bees occupying a Langstroth honeycomb with a brood chamber on both sides (2,430 bees) were used (Delaplane et al., 2013). The percentage surface of brood, honey and pollen was converted into area (cm2), using the surface that has a Langstroth-type honeycomb on both sides (1,760 cm2) (Delaplane et al., 2013). Measurements were made during the afternoon period (4 to 7 p.m.) when most of the bees were inside the hives. The weight of the colonies was determined by subtracting the weight of the equipment (floor, brood chamber, roof and combs) from the total weight of each hive.
The evaluations were carried out with a monthly frequency from May 2016 to April 2017. 1.5 L of sugar syrup (1: 1) was supplied weekly and 250 g of protein substitute (25% protein) every two weeks, when the Environmental conditions did not provide food to the colonies (June 14 to July 22 and from December 10, 2016 to January 20, 2017).
Infestation by V. destructor. The level of infestation in adult bees, in the brood of workers and the number of fallen mites in the experimental colonies, was determined every month from May 2016 to April 2017. The level of infestation in bees was determined by the method of De Jong by dividing the number of mites by the total number of bees analyzed and multiplied by 100 (De Jong et al., 1982). The total number of mites in the adult bees of the colonies was estimated with the average number of mites per bee multiplied by the estimated bee population in the colonies (Delaplane et al., 2013). The level of infestation in the worker brood was determined by dividing the number of infested cells in a portion (10 x 10 cm) of honeycomb with capped brood by the number of cells analyzed and multiplying by 100 (De Jong et al., 1982).
To register fallen mites, a galvanized sheet (28 x 43.5 cm) impregnated with petrolatum was installed on the floor of the hives and a mesh (3 mm) was placed between the sheet and the brood chamber, this so that the mites fallen through the mesh and adhere to the sheet. The daily average of fallen varroas was obtained by dividing the number of registered mites by seven days that the adherent sheets remained in place (Dietemann et al., 2013). In addition, during the application of the acaricidal treatment, the fall of mites in the sheets of the four groups of colonies was recorded weekly for four weeks.
Statistical analysis. From the monthly records, the average of each of the measured variables (bee population, brood areas (cm2), honey and pollen, weight, levels of infestation in adult bees, in brood of workers and the number of fallen mites) for each season of the year, and analysis of variance tests, repeated measures and the Newman-Keuls mean comparison test were used. In addition to Pearson's correlation tests to establish relationships between the evaluated variables and the X 2 test to determine possible differences in the frequency of colony mortality cases between colonies treated with amitraz in summer, summer and winter, winter and control. The percentage data were transformed to the square root of the arcsine, this to normalize its distribution (SAS, 2014).
RESULTS
At the beginning of the experiment (April 2016), the 48 selected colonies had statistically similar conditions of bee population (F=0.14, P=0.35), capped brood (F=0.42, P=0.52), honey reserves (F=0.14, P=0.78), pollen (F=0.39, P = 0.65) and infestation levels of V. destructor (F = 0.44, P = 0.51).
A significant reduction in the varroa population was observed because of the acaricidal treatments applied in summer and winter. The values of the levels of infestation in adult bees, brood and the daily fall of varroas before and after the treatments applied in summer and winter are shown in tables 1 and 2.
Table 1 Level of infestation by V. destructor in bees and breeding (% mean±se) and daily fall (mean±se) of varroas before and after treatment with amitraz in the summer, of colonies of the experimental groups: summer, winter, summer and winter and control.
| Variable/group of colonies | Summer | Winter | Summer and winter | Control | F and P |
|---|---|---|---|---|---|
| Adult bee infestation before treatment | 7.01±0.66a | 10.51±2.4a | 10.95±1.33a | 10.36±1.69a | 1.42, 0.25 |
| Adult bee infestation after treatment | 0.69±0.44b | 13.98±2.7a | 0.94±0.50b | 11.05±1.83a | 18.97, <0.0001 |
| Infestation in brood before treatment | 11.0±1.75a | 8.40±1.84a | 10.09±2.23a | 10.82±1.54ª | 0.39, 0.76 |
| Infestation in brood after treatment | 2.31±0.57b | 25.25±4.73a | 5.25±1.45b | 19.34±2.58a | 17.68, <0.0001 |
| daily fall of varroa before treatment | 51.13±9.2a | 77.0±15a | 72.7±10a | 63.9±11a | 1.01, 0.39 |
| Daily loss of varroa after treatment | 38.2±3.7b | 66.7±14.8ab | 36.9±4b | 113.58±31a | 4.46, 0.008 |
Untransformed values. Different literals between rows indicate significant differences based on an analysis of variance and the comparison of means with the Newman-Keuls test, after data transformation
Table 2 Level of infestation by V. destructor in bees and brood (% mean ± se) and daily fall (mean ± se) of varroas before and after treatment with amitraz in winter, of colonies of the experimental groups: summer, winter, summer and winter and control.
| Variable/group of colonies | Summer | Winter | Summer and winter | Control | F and P |
|---|---|---|---|---|---|
| Adult bee infestation before treatment | 10.77±1.07a | 10.43±1.69a | 9.41±1.48a | 9.73±1.42a | 0.22, 0.88 |
| Adult bee infestation after treatment | 5.68±0.72a | 0.09±0.04b | 0.47±0.33b | 7.2±1.22a | 18.97, <0.0001 |
| Infestation in brood before treatment | 7.88±1.25a | 16.22±2.24a | 10.4±2.2a | 14.1±3.63a | 2.55, 0.070 |
| Infestation in brood after treatment | 0.62±0.24b | 0.45±0.30b | 0.20±0.10b | 2.20±0.85a | 2.89, 0.045 |
| daily fall of varroa before treatment | 20.7±4.3a | 16.8±4.2a | 21.3±3.6a | 17.6±3.5a | 0.29, 0.82 |
| Daily loss of varroa after treatment | 1.75±0.48b | 1.31±0.30b | 1.36±0.25b | 3.8±0.9a | 4.35, 0.01 |
Untransformed values. Different literals between rows indicate significant differences based on an analysis of variance and the comparison of means with the Newman-Keuls test, after data transformation.
When using the monthly records of the levels of infestation by Varroa and generating an average for each season of the year, it was observed that these differ significantly between the four groups of colonies (P.05). The treatment applied in the summer reduced the levels of infestation in adult bees, brood and the fall of mites to the adherent bottom of the hives. However, in adult bees, infestation levels increased rapidly by fall to levels similar to those at which they were initially treated (figure 1).
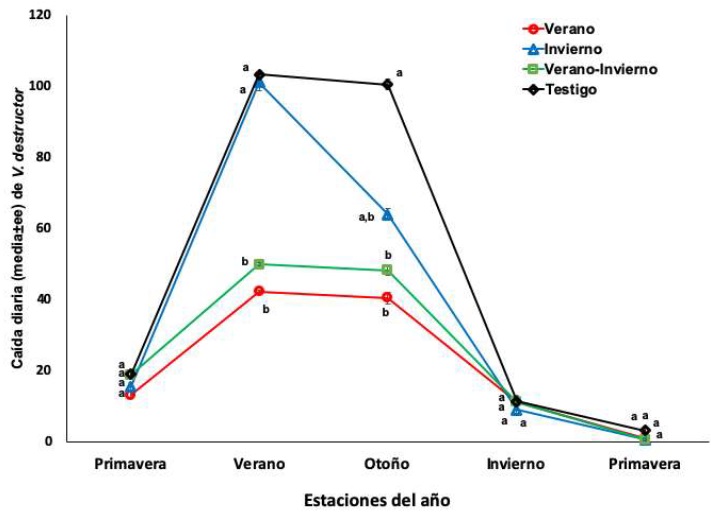
Figure 1 Infestation level (mean±ee) of V. destructor in adult bees from colonies treated with amitraz in summer, winter, summer and winter and not treated. Untransformed values. Different literals indicate significant differences (P <0.05) based on an analysis of repeated measurements and the comparison of means with the Newman-Keuls test, after data transformation to the arcsine of the square root.
The Varroa population in the breeding and the mites fallen on the adherent floor of the hives had a similar behavior (figures 2 and 3). In both sites, the colonies treated in summer (summer and summer and winter) presented significantly levels (F=7.01, P=0.0008) lower in the summer and autumn seasons, as opposed to the colonies treated in the winter and untreated. The acaricidal treatment applied in winter only had an effect on the mite population in adult bees. The number of mites on the sticky bottom and on the brood during winter and spring did not differ between the four groups of colonies (Figures 1, 2 and 3).

Figure 2 Infestation level (mean ± se) of V. destructor in colony rearing treated with amitraz in summer, winter, summer-winter and control. Untransformed values. Different literals indicate significant differences (P <0.05) based on a repeated measurements analysis with transformed values and comparison of means with the Newman-Keuls test.
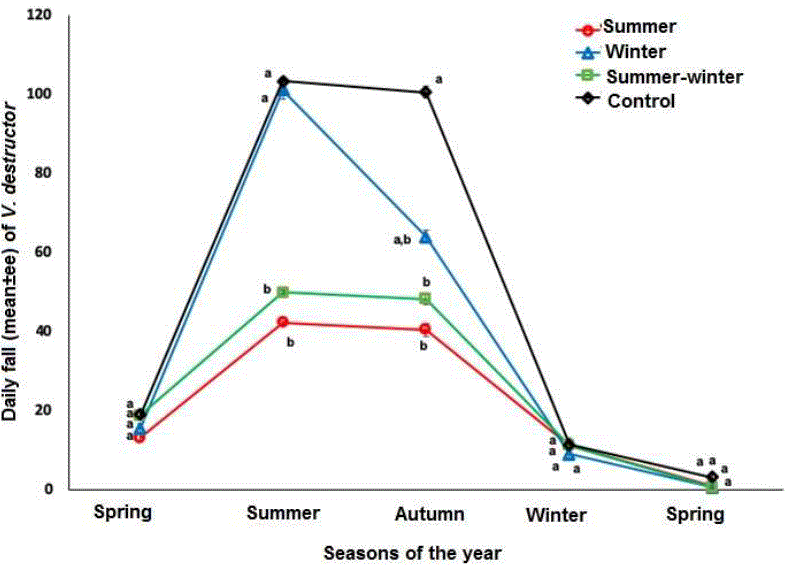
Figure 3 Daily fall (mean±se) of V. destructor in colonies treated with amitraz in summer, winter, summer-winter and control. Different literals indicate significant differences based on a repeated measures analysis and comparison of means with the Newman-Keuls test (P <0.05).
Regarding the fall of varroas on the adherent floor of the hives during the four applications of the acaricide treatment, it was observed that the first application of the treatment caused significantly (F = 8.51, P <0.001) a greater number of fallen mites than in subsequent applications. The fall of mites due to the last three applications of amitraz were statistically similar (P> 0.05) to the natural fall of the mite of the control group.
The bee population, honey areas, pollen and weight were not different between the four groups of colonies in the four seasons of the year. Significant differences were only observed in the capped brood areas during the fall, the colonies treated in summer (6,747 ± 352 cm2) and summer-winter (5,960 ± 191 cm2) had significantly (F = 3.55, P = 0.023), larger areas of brood than the colonies treated in the winter (4,889 ± 472 cm2) or untreated (5,240 ± 631 cm2), between which there was no significant difference.
Without considering the treatment to which the colonies belonged, the capped brood areas and the bee population were statistically lower in the last two seasons of the experiment (winter and spring), unlike what was observed in the first seasons, these values are presented in Table 3.
Table 3 Areas (cm2) of capped brood and adult bee population of honeybee colonies (n=48) during spring, summer, autumn and winter of 2016 and spring of 2017.
| Seasons | Breeding areas (cm2) | Bee population |
|---|---|---|
| Spring (2016) | 10,410±182 a | 9,484±136 a,b |
| Summer (2016) | 8,387±220 b | 10,011±398 a |
| Autumn (2016) | 5,771±238 c | 8,614±417 b |
| Winter (2016) | 3,252±240 d | 4,366±372 c |
| Spring (2017) | 5,813±275 c | 5,123±445 c |
| F and P | F=146.8, P<0.0001 | F=48.9, P<0.0001 |
Different literals in each column indicate significant differences based on an analysis of variance and comparison of means with the Newman-Keuls test.
At the end of the experiment (spring), the average number of varroa mites per bee and the estimated population size of varroa mites in the adult bee population of the colonies was significantly lower in the groups treated in winter (winter and summer and winter). There were no significant differences regarding the population values, food reserves and weight between the colonies treated in summer, winter, summer and winter and the control group (Table 4).
Table 4 Bee population, brood areas (cm2), honey, pollen, weight, average varroa per bee and mite population in adult bees from colonies treated with amitraz in the summer, winter, summer-winter and control, at the end of the experiment (spring).
| Group | Bee population | Breeding Populatio n (cm2) | Honey reserves (cm2) | Pollen reserves (cm2) | Weight (Kg) | Varroas per 100 bees | Mites in estimated bee population |
|---|---|---|---|---|---|---|---|
| Summer | 13314±953 | 5560±475 | 3560±372 | 660±220 | 8.5±1.02 | 0.045±0.0 a | 602±114 a |
| Winter | 12757±1349 | 6356±464 | 3276±544 | 605±321 | 6.2±1.03 | 0.0086±0.01 b | 109±50 b |
| Summer | 12879±1542 | 5808±593 | 3036±266 | 968±205 | 7.3±0.81 | 0.0078±0.00 b | 100±42 b |
| -winter | 14200±1847 | 5555±743 | 3410±360 | 1320±525 | 6.7±0.85 | 0.0293±0.01 a | 416±86 a |
| Control | F=0.19, | F=0.43, | F=0.34, | F=1.01, | F=1.13, | F=10.09, | F=8.71, |
| P=0.90 | P=0.73 | P=0.79 | P=0.39 | P=0.35 | P<0.0001 | P=0.0002 |
Different literals in each column indicate significant differences based on an analysis of variance and comparison of means with the Newman-Keuls test.
The level of infestation in bees was positively and significantly related to the level of infestation in brood (r = 0.52, P = 0.0002) and with the fall of mites to the adherent floor of the hives (r = 0.63, P <0.0001). Likewise, the weight of the colonies was related to the bee population of the colonies (r = 0.65, P <0.001).
In the group of colonies treated in the summer 8% of the colonies died and 16% in the group treated in the summer and winter, while in the group treated in the winter and control there was a mortality of 25% and 33% , respectively, there were no statistically significant differences (X 2 = 2.52, gl = 3, P = 0.47).
DISCUSSION
The reduction in the varroa population because of the treatments applied in summer and winter confirm the acaricidal effectiveness of amitraz by significantly reducing the population of mites in bee colonies (Semkiw et al., 2013). However, a rapid increase in the levels of infestation in adult bees was observed in the fall in the colonies treated during the summer. It has been presented in other investigations and has been attributed to varroa survivors of treatment and the migration of mites from of untreated colonies from the same apiary (Delaplane and Hood, 1997; Gatien and Currie, 2003; Wilfert et al., 2016). Considering the infestation averages for each season of the year, it was observed that the acaricidal treatment applied in winter only had an effect on the population of mites in adult bees (≈7.3% less compared to the levels of infestation in autumn). The number of mites on the sticky bottom in the hives (mean = 10.5) and in the brood (mean = 10.1%) during winter and spring (1.4 and 2.5%, respectively), did not differ (P>0.05) between the four groups of colonies. In the few studies with which the present results can be compared, variability is observed in the results and some coincide with those obtained in the present study, such is the case of the experiment carried out by Delaplane and Hood (1997). They did not find a reduction in the number of mites in the colonies treated in June (1,702 varroas) compared to the untreated colonies (986), this in the state of Georgia USA. However, in this study they found that for South Carolina the effect of the treatment on the mite population was significant.
The population dynamics of V. destructor is related to the cycle and the amount of young available in the colony (Delaplane, 1998; Caron, 1999). Moreover, the increase in the population of bees and brood reduces the proportion of mites with respect to the population of bees (Moretto et al., 1991). This explains the notable reduction in the levels of infestation at the end of the experiment in the four groups of colonies, since there was a significant reduction in the breeding areas and bee population in autumn and winter (P <0.0001, table 3), which caused less possibilities of reproduction for the mite. Additionally, in the spring, the increase observed in the breeding areas and bee population derived from a greater availability of resources for bees could reduce the proportion of varroas with respect to the bee population.
In relation to the number of applications of the treatment, the results reveal that in the first dose there was a greater (P <0.001) fall of mites with respect to the subsequent applications and the control group. This is probably because most of the mites die during the first application and the small number of remaining varroas in the phereal and reproductive phase does not allow the identification of significant differences with respect to the control group in subsequent applications of the treatment. These results coincide with those previously reported with the use of thymol (Espinosa-Montaño and Guzmán-Novoa, 2007). However, it is important to maintain the treatment during the emergence of new varroas that are in the reproductive stage inside the cells, this will allow a better reduction of the mite population in the colonies (Rosenkranz et al., 2010).
The bee population, honey areas, pollen and weight were not different between the four groups of colonies in the four seasons of the year. Significant differences were only observed in the capped brood areas during the autumn, the colonies treated in summer (6,747 ± 352 cm2) and summer and winter (5,960 ± 191 cm2) had significantly (P = 0.023), larger brood areas than the colonies treated in winter (4,889 ± 472 cm2) or untreated (5,240 ± 631 cm2), between which there was no significant difference.
With the exception of the treatments applied in the summer, which had repercussions with larger breeding areas in the fall (P = 0.023). The differences in the levels of Varroa infestation between the treatments (colonies treated in summer, winter, summer-winter and control) did not have significant effects on the population variables (bees and breeding areas), food reserves (honey and pollen) and weight of the colonies.
As in the present study, the works carried out by Delaplane and Hood (1997,1999) and Strange and Sheppard (2001) show contradictory results on the effect of infestation levels of V. destructor on the population parameters of the colonies, their food reserves and the weight of the colonies. In this regard, it has been reported that there is not always a clear relationship between the mite population and the colony population (Korpela et al., 1992) and that acaricidal treatments sometimes do not affect the production of offspring in colonies parasitized by Varroa (Delaplane, 1995). This is probably due to increased brood production in colonies with high levels of Varroa infestation due to the efforts of the colonies to compensate for the loss of offspring because of parasitosis (Delaplane and Hood, 1997).
At the end of the experiment (spring), the average infestation levels of 0.045 mites per bee (4.5% infestation) and an estimated population of 100 to 602 mites did not reduce the bee population, brood areas, honey, pollen and weight of the colonies of the central highlands of Mexico with populations of 12,757 to 14,200 bees. In this sense, it has been reported that the tolerable infestation level for the colony changes regionally due to the variation in the breeding period in the colonies and its effects on the population dynamics of the mite (Delaplane, 1998; Caron, 1999).
In Mexico, SAGARPA (2005) recommends that after 5% Varroa infestation in bees and/or 10 mites fallen in 24 h on the adherent floor of hives; some treatment against the disease must be implemented. However, these thresholds differ from others established by different authors in different times and regions. The treatment threshold established for the northwestern and southwestern US is 5% in bees and 12 mites fallen in 24 hours in spring (Strange and Sheppard, 2001). For the summer it is recommended that the acaricidal treatment be applied from 23 mites to the northwest and 70 to 224 fallen in 24 h or from 3,000 to 4,000 mites for colonies with populations of 24,000 to 34,000 bees (8- 8.5% in bees) in the southeastern US (Delaplane and Hood, 1997,1999). In contrast, for the United Kingdom the damage threshold has been reported as 2,500 mites per colony (Martin, 1999).
In Canada, the average levels of infestation by Varroa of 2% in bees have a negative effect on honey production and in colonies with levels above 4% in summer, it is necessary to apply a treatment to prevent its loss in autumn and winter (Currie and Gatien, 2006). Contradictorily, Currie and Gatien (2006) report that levels of 7 and 10% in bees do not affect the honey production of the colonies. In Valle de Bravo, state of Mexico, it was observed that colonies treated with fluvalinate and with an average infestation of 2.3% produced 65% more honey than untreated colonies and with an average level of infestation of 6.8% (Arechavaleta and Guzmán-Novoa, 2000). The above reflects the variability of effects that the levels of infestation exert on the honeybee colonies in different environmental conditions and times of the year. Probably, the presence of viruses and other health factors could explain the variations found in the reported treatment thresholds.
The correlation analysis allowed to observe a positive relationship between the levels of infestation in bees and brood and with the daily fall of mites, as well as the weight with the population of bees in the colonies. The foregoing coincides with that reported by Gąbka (2014) and Saini (2018). These characteristics can be used by beekeepers as predictive strategies for colony conditions.
Colonies that receive acaricidal treatment at the beginning of the year have been reported to have a lower mortality rate than colonies treated late or not treated (Delaplane and Hood, 1997; Fries et al., 2006). However, in the present study the mortality rate of the four groups of colonies did not differ significantly.
Additional studies are required to provide more information regarding the effect of Varroa treatment applied in different seasons of the year on the levels of mite infestation and population and feeding conditions, as well as its effect on the production of honeybee colonies in different regions.
CONCLUSION
The treatment applied in the summer significantly reduced the levels of Varroa infestation, but these increased for the fall to similar levels to the initial ones. The differences in the levels of infestation observed in autumn, winter and spring did not affect the population of bees, breeding areas, honey, pollen and weight of the colonies. Average infestation levels of 4.5% in bees and an estimated population of up to 602 mites did not reduce the population, food reserves and weight of the colonies evaluated in the present study with populations of up to 14,200 bees. It is recommended to monitor the infestation level of the colonies and apply acaricides during the winter to prevent the mite population from increasing and having an impact on the conditions and productivity of the bee colonies.
ACKNOWLEDGEMENT
We thank the Academic Unit of Veterinary Medicine and Zootechnics of the Autonomous University of Zacatecas, the College of Postgraduates Campus San Luis Potosí for the facilities granted to carry out this study and obtain the degree of Master of Science in Innovation in Management of Natural Resources of the first author, as well as CONACYT for the grant awarded.
REFERENCES
Anderson DL, Trueman JWH. 2000. Varroa jacobsoni (Acari: Varroidae) is more than one species. Experimental and Applied Acarology. 24(3):165-189. ISSN: 1572-9702. https://doi.org/10.1023/A:1006456720416 [ Links ]
Arechavaleta VME, Guzmán-Novoa E. 2000. Producción de miel en colonias de abejas (Apis mellifera L.) tratadas y no tratadas con fluvalinato contra Varroa jacobsoni Oudemans en Valle de bravo, Estado de México. Veterinaria México. 31(4):381-384.ISSN 2448-6760. https://www.medigraphic.com/pdfs/vetmex/vm-2000/vm004m.pdf [ Links ]
Berry JA, Hood WM, Pietravalle S, Delaplane KS. 2013. Field-Level Sublethal Effects of Approved Bee Hive Chemicals on Honey Bees (Apis mellifera L). PLoS ONE. 8(10): e76536. ISSN: 1932-6203. https://doi.org/10.1371/journal.pone.0076536 [ Links ]
Boncristiani H, Underwood R, Schwarz R, Evans JD, Pettis J, vanEngelsdorp D. 2012. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. Journal of Insect Physiology. 58:613-620. ISSN: 00221910. https://doi.org/10.1016/j.jinsphys.2011.12.011 [ Links ]
Caron D. 1999. Delaware bee mites survey. American Bee Journal. 139(8): 631-633. ISSN: 00027626. https://europepmc.org/article/agr/ind22002953?client=bot&client=bot&client=bot [ Links ]
Currie RW, Gatien P. 2006. Timing acaricide treatments to prevent Varroa destructor (Acari: Varroidae) from causing economic damage to honey bee colonies. The Canadian Entomologist. 138(2):238-252. ISSN: 0008-347X. https://doi.org/10.4039/n05-024 [ Links ]
Dainat B, Evans JD, Chen YP, Gauthier L, Neumann P. 2012. Dead or alive: deformed wing virus and Varroa destructor reduce the life span of winter honeybees. Applied Environmental Microbiology. 78(4):981-987. ISSN: 1098-5336. http://dx.doi.org/10.1128/AEM.06537-11 [ Links ]
Delaplane KS, Der-Steen JV, Guzman-Novoa E. 2013. Standard methods for estimating strength parameters of Apis mellifera colonies. Journal of Apicultural Research. 52(1):1-12. ISSN: 0021-8839. https://doi.org/10.3896/IBRA.1.52.1.03 [ Links ]
Delaplane KS, Hood WM. 1997. Effects of delayed acaricide treatment in honey bee colonies parasitized by Varroa jacobsoni and a late-season treatment threshold for the south-eastern USA. Journal of Apicultural Research. 36(3/4):125-132. ISSN: 0021-8839. https://doi.org/10.1080/00218839.1997.11100938 [ Links ]
Delaplane KS, Hood WM. 1999. Economic threshold for Varroa jacobsoni Oud. in the southeastern USA. Apidologie. 30: 383-395. ISSN: 0044-8435. https://doi.org/10.1051/apido:19990504 [ Links ]
Delaplane KS. 1995. Effects of Terramycin antibiotic and Apistan acaricide on colonies of honey bees (Hymenoptera: Apidae) infested with Varroa jacobsoni (Parasitiformes: Varroidae). Journal of Economic Entomology. 88(5):1206-1210. ISSN 0022-0493. https://doi.org/10.1093/jee/88.5.1206 [ Links ]
Delaplane KS. 1998. Varroa control: timing is everything. American Bee Journal. 138:575-576. ISSN: 0002-7626. https://agris.fao.org/agris- search/search.do?recordID=US1997076691 [ Links ]
Dietemann V, Nazzi F, Martin SJ, Anderson D, Locke B, Delaplane K, Wauquiez Q, Tannahill C, Frey E, Ziegelmann B, Rosenkranz P, Ellis JD. 2013. Standard methods for varroa research. Journal of Apicultural Research. 52(1):1-54. ISSN: 0021-8839. https://doi.org/10.3896/ibra.1.52.1.09 [ Links ]
De Jong D, Roma DA, Goncalves LS. 1982. A comparative analysis of shaking solutions for the detection of Varroa jacobsoni on adult honeybees. Apidologie. 13:297-306. ISSN: 1297-9678. https://www.apidologie.org/articles/apido/pdf/1982/03/Apidologie_0044-84351982133ART0008.pdf [ Links ]
Espinosa-Montaño LG, Guzmán-Novoa E. 2007. Eficacia de dos acaricidas naturales, ácido fórmico y timol, para el control del ácaro Varroa destructor de las abejas (Apis mellifera L.) en Villa Guerrero, Estado de México. Veterinaria México38(1):9-19. ISSN: 0301-5092. http://www.redalyc.org/articulo.oa?id=42338102 [ Links ]
Fries I, Imdorf A, Rosenkranz P. 2006. Survival of mite infested (Varroa destructor) honey bee (Apis mellifera) colonies in a Nordic climate. Apidologie. 37:564-570. ISSN: 0044-8435. https://doi.org/10.1051/apido:2006031 [ Links ]
Gąbka J. 2014. Correlations between the strength, amount of brood and honey production of the honey bee colony. Medycyna Weterynaryjna. 70(12):754-756. ISSN: 0025-8628. https://www.researchgate.net/profile/Jakub_Gbka/publication/293074876_Correlations_between_the_strength_amount_of_brood_and_honey_production_of_the_honey_bee_colony/links/58385ea008ae3d91723dd7f7/Correlations-between-the-strength-amount-of-brood-and-honey-production-of-the-honey-bee-colony.pdf [ Links ]
Gashout HA, Goodwin PH, Guzman-Novoa E. 2018. Lethality of synthetic and natural acaricides to worker honey bees (Apis mellifera) and their impact on the expression of health and detoxification-related genes. Environmental Science and Pollution Research. 25(34): 34730-34739. ISSN: 09441344. https://doi.org/10.1007/s11356-018-3205-6 [ Links ]
Gashout HA, Guzman-Novoa E, Goodwin PH, Correa-Benítez A. 2020. Impact of sublethal exposure to synthetic and natural acaricides on honey bee (Apis mellifera) memory and expression of genes related to memory. Journal of Insect Physiology. 121 104014. ISSN: 00221910. https://doi.org/10.1016/j.jinsphys.2020.104014 [ Links ]
Gatien P, Currie RW. 2003. Timing of acaracide treatments for control of low-level populations of Varroa destructor (Acari: Varroidea) and implications for colony performance of honey bee. The Canadian Entomologist. 135(5):749-763. ISSN: 0008-347X. https://doi.org/10.4039/n02-086 [ Links ]
González-Cabrera J, Rodríguez-Vargas S, Davies TGE, Field LM, Schmehl D, Ellis JD, Krieger K, Williamson MS. 2016. Novel Mutations in the Voltage-Gated Sodium Channel of Pyrethroid-Resistant Varroa destructor Populations from the Southeastern USA. PLoS ONE. 11(5): e0155332. ISSN: 1932-6203. https://doi.org/10.1371/journal.pone.0155332 [ Links ]
Gregorc A, Planinc I. 2012. Use of thymol formulations, amitraz, and oxalic acid for the control of the varroa mite in honey bee (Apis mellifera carnica) colonies. Journal of Apicultural Science. 56(2): 61-69. ISSN: 2299-4831. https://doi.org/10.2478/v10289-012-0024-8 [ Links ]
Guzmán-Novoa E, Eccles L, Calvete Y, McGowan J, Kelly PG, Correa-Benítez A. 2010. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie. 41(4): 443-450. ISSN: 0044-8435. https://doi.org/10.1051/apido/2009076 [ Links ]
INEGI. 2019. Prontuario de información geográfica municipal de los Estados Unidos Mexicanos. Instituto Nacional de Estadística Geografía e Informática. Consultado el 8 de junio de 2019 en: https://www.inegi.org.mx/app/areasgeograficas/?ag=32 [ Links ]
Kamler M, Nesvorna M, Stara J, Erban T, Hubert J. 2016. Comparison of taufluvalinate, acrinathrin, and amitraz effects on susceptible and resistant populations of Varroa destructor in a vial test. Experimental and applied acarology. 69(1): 1-9. https://doi.org/10.1007/s10493-016-0023-8 [ Links ]
Korpela S, Aarhus A, Fries I, Hansen H. 1992. Varroa jacobsoni Oud. in cold climates: population growth, winter mortality and influence on the survival of honey bee colonies. Journal of Apicultural Research. 31(3/4): 157-164. ISSN: 0021-8839. https://doi.org/10.1080/00218839.1992.11101278 [ Links ]
Lupo A, Gerling D. 1990. A comparison between the efficiency of summer treatments using formic acid and Taktic® against Varroa jacobsoni in beehives. Apidologie. 21(3): 261-267. ISSN: 1297-9678.https://doi.org/10.1051/apido:19900311 [ Links ]
Marie-Pierre C, Anne-Claire M, Zeggane S, Drajnudel P, Schurr F, Marie-Claude C, Ribière-Chabert M, Aubent M, Jean-Paul F. 2010. A case control study and a survey on mortalities of honey bee colonies (Apis mellifera) in France during the winter of 2005-6. Journal of Apiculture Research. 49(1):40-51. ISSN: 0021-8839. https://doi.org/10.3896/IBRA.1.49.1.06 [ Links ]
Martin SJ, Ball BV, Carreck NL. 2010. Prevalence and persistence of deformed wing virus (DWV) in untreated or acaricide-treated Varroa destructor infested honey bee (Apis mellifera) colonies. Journal of Apicultural Research. 49(1): 72-79. ISSN: 0021-8839. https://doi.org/10.3896/IBRA.1.49.1.10 [ Links ]
Martin SJ. 1999. Population modeling and the production of a monitoring tool for Varroa jacobsoni an ectoparasitic mite of honey bees. Aspects of Applied Biology. 53: 105-112. ISSN: 0265-1491. https://www.researchgate.net/publication/291772610_Population_modelling_and_the_production_of_a_monitoring_tool_for_Varroa_jacobsoni_an_ectoparasitic_mite_of_honey_bees#fullTextFileContent [ Links ]
Martínez-Puc JF, Medina-Medina LA. 2011. Evaluación de la resistencia del ácaro Varroa destructor al fluvalinato en colonias de abejas (Apis mellifera) en Yucatán, México. Revista Mexicana de Ciencias Pecuarias. 2(1):93-100. ISSN 2448-6698. https://cienciaspecuarias.inifap.gob.mx/index.php/Pecuarias/article/view/1451 [ Links ]
Medina-Flores CA, Guzmán-Novoa E, Aréchiga-Flores CF, Aguilera-Soto JI, Gutiérrez-Piña J. 2011. Efecto del nivel de infestación de Varroa destructor sobre la producción de miel de colonias de Apis mellifera en el altiplano semiárido de México. Revista Mexicana de Ciencias Pecuarias. 2:313-317. ISSN 2448-6698. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-11242011000300006 [ Links ]
Moretto G, Goncalves SL, De Jong D. 1991. Africanized bees are more efficient at removing Varroa jacobsoni-Preliminary data. American Bee Journal. 131(7):434. ISSN: 0002-7626. https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=19855220 [ Links ]
Nazzi F, Le Conte Y. 2016. Ecology of Varroa destructor, the major ectoparasite of the western honey bee, Apis mellifera. Annual Review of Entomology. 61:417-432. ISSN: 1545-4487. https://doi.org/10.1146/annurev-ento-010715-023731 [ Links ]
Pietropaoli M, Formato G. 2018. Liquid formic acid 60% to control varroa mites (Varroa destructor) in honey bee colonies (Apis mellifera): protocol evaluation. Journal of Apicultural Research. 57(2): 300-307. ISSN: 0021-8839. https://doi.org/10.1080/00218839.2017.1376767 [ Links ]
Rodríguez-Dehaibes SR, Otero-Colina G, Villanueva-Jiménez JA, Corcuera P. 2011. Susceptibility of Varroa destructor (Gamasida: Varroidae) to four pesticides used in three mexican apicultural regions under two different management systems. International Journal of Acarology. 37(5): 441-447. ISSN: 1572-9702.https://doi.org/10.1080/01647954.2010.525523 [ Links ]
Rosenkranz P, Aumeier P, Ziegelmann B. 2010. Biology and control of Varroa destructor. Journal of invertebrate pathology. 103:S96-S119. ISSN: 1096-0805 https://doi.org/10.1016/j.jip.2009.07.016 [ Links ]
Ryabov EV, Childers AK, Chen Y, Madella S, Nessa A, Evans JD. 2017. Recent spread of Varroa destructor virus-1, a honey bee pathogen, in the United States. Scientific reports. 7(1):1-10. ISSN: 2045-2322. https://doi.org/10.1038/s41598- 017-17802-3 [ Links ]
SAGARPA (Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación). 2005. Modificación a la Norma Oficial Mexicana NOM-001-ZOO-1994, campaña contra la varroasis de las abejas. Diario Oficial. 28 de diciembre de 2005. http://www.ordenjuridico.gob.mx/Federal/PE/APF/APC/SAGARPA/Modificaciones/28122005(1).pdf [ Links ]
Saini S, Chaudhary OP, Anoosha, V. 2018. Relationship of population size and extraction frequency with honey production in Apis mellifera colonies. Journal of Entomology and Zoology Studies. 6(3):1374-1377. ISSN: 2320-7078.https://www.researchgate.net/profile/Op_Chaudhary2/publication/328902070_Relations hip_of_population_size_and_extraction_frequency_with_honey_production_in_Apis_mel lifera_colonies/links/5bea992d299bf1124fce68f2/Relationship-of-population-size-and- extraction-frequency-with-honey-production-in-Apis-mellifera-colonies.pdf [ Links ]
SAS Intitute. 2014. Statistical Analysis Software SAS/STAT®. version 9.4, Cary, N.C., USA: SAS Institute Inc., ISBN: 978-1-60764-599-3.https://www.sas.com/en_us/software/stat.html [ Links ]
Schneider S, Eisenhardt D, Rademacher E. 2012. Sublethal effects of oxalic acid on Apis mellifera (Hymenoptera: Apidae): changes in behaviour and longevity. Apidologie. 43(2): 218-225. ISSN: 0044-8435.https://doi.org/10.1007/s13592-011-0102-0 [ Links ]
Smodiš ŠMI, Nakrst M, Žvokelj L, Gregorc A. 2011. The acaricidal effect of flumethrin, oxalic acid and amitraz against Varroa destructor in honey bee (Apis mellifera carnica) colonies. Acta Veterinaria Brno. 80(1): 51-56. ISSN: 1801-7576. https://doi.org/10.2754/avb201180010051 [ Links ]
Semkiw P, Skubida P, Pohorecka K. 2013. The amitraz strips efficacy in control of Varroa destructor after many years application of amitraz in apiaries. Journal of Apicultural Science. 57(1): 107-121. ISSN: 2299-4831. https://content.sciendo.com/view/journals/jas/57/1/article-p107.xml [ Links ]
Strange JP, Sheppard WS. 2001. Optimum timing of miticide applications for control of Varroa destructor (Acari: Varroidae) in Apis mellifera (Hymenoptera: Apidae) in Washington State, USA. Entomological Society of America94(6):1324-1331. ISSN 0022-0493. https://doi.org/10.1603/0022-0493-94.6.1324 [ Links ]
Vanengelsdorp D, Meixner MD. 2010. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. Journal of Invertebrate Pathology. 103: S80-S95. ISSN: 0022-2011.https://doi.org/10.1016/j.jip.2009.06.011 [ Links ]
Wilfert L, Long G, Leggett HC, Schmid-Hempel P, Butlin R, Martin SJM, Boots M. 2016. Deformed wing viruses is a recent global epidemic in honeybees driven by Varroa mites. Research Report Science. 351(6273):594-597. ISSN 1095-9203. https://doi.org/10.1126/science.aac9976 [ Links ]
Received: April 27, 2020; Accepted: November 26, 2020











 text in
text in 


