Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Abanico veterinario
versión On-line ISSN 2448-6132versión impresa ISSN 2007-428X
Abanico vet vol.10 Tepic ene./dic. 2020 Epub 02-Mar-2021
https://doi.org/10.21929/abavet2020.9
Original article
Ruminal lesions in cattle slaughtered in slaughterhouses
1Maestría Interinstitucional en Producción Pecuaria. Universidad de Guanajuato. México.
2Departamento de Veterinaria y Zootecnia, División de Ciencias de la Vida, Universidad de Guanajuato, Irapuato, Guanajuato, México.
3Facultad de Medicina Veterinaria y Zootecnia, Universidad de Colima, México.
4Centro de Enseñanza, Investigación y Extensión en Producción Animal en Altiplano, Tequisquiapan, Querétaro. México.
The objective of the present study was to describe macroscopic and microscopic lesion in the rumen of bovine slaughtered in the Municipal Meat Processor of Colima, Mexico. The lesions found during the slaughter were macroscopically and subsequently fixed in 10% buffered formalin with pH 7.2 described. It was with the routine histological technique, and stained with hematoxylin-eosin (H-E) processed. 100 rumen were collected, as well as data on the origin, age, sex, and species of the animals. Data were, with the Chi- square independence test and logistic regression analysis analyzed. The 98% of the bovines presented at least one of the following lesions: hemorrhages, erosions, ulcers, scars, hyperkeratosis, hydropic degeneration, rumenitis, lymphangiectasia, a sample had a papilloma and the presence of the protozoan Balantidium coli was in 30% of the samples found. Dependence was found between B. coli to the origin (P= 0.046) and the sex (P= 0.041) of the animals. The variables that were significant (P= <0.05) in the logistic regression analysis were scars, rumenitis, lymphangiectasia, and B. coli. The injuries found to interfere with the absorption of nutrients, loss of production, and productivity, as well as the presence of opportunistic pathogens that can represent a health risk for animals and humans.
Keywords: hyperkeratosis; rumenitis; Balantidium coli; ruminal acidosis
El objetivo del presente estudio fue describir los hallazgos macroscópicos y microscópicos encontrados en el rumen de bovinos sacrificados en la Procesadora Municipal de Carne de Colima, Colima, México. Las lesiones encontradas durante la faena se describieron macroscópicamente, posteriormente se fijaron en formalina amortiguada al 10% con pH 7.2, se procesaron con la técnica histológica de rutina y se tiñeron con hematoxilina-eosina (H-E). Se colectaron 100 rúmenes, así como datos de la procedencia, edad, sexo y especie de los animales. La información fue analizada con la prueba de independencia de Chi cuadrada y un análisis de regresión logística. El 98% de los bovinos presentaron al menos una de las siguientes lesiones: hemorragias, erosiones, úlceras, cicatrices, hiperqueratosis, degeneración hidrópica, rumenitis, linfangiectasia, una muestra presentó un papiloma y se encontró la presencia del protozoario Balantidium coli en el 30% de las muestras. Se encontró dependencia entre B. coli con la procedencia (P=0.046) y el sexo (P=0.041) de los animales. Las variables que fueron significativas (P=<0.05) en el análisis de regresión logística fueron las cicatrices, rumenitis, linfangiectasia y B. coli. Las lesiones encontradas interfieren con la absorción de nutrientes, pérdida de producción y productividad, así como la presencia de patógenos oportunistas que pueden representar un riesgo sanitario para animales y humanos.
Palabras clave: hiperqueratosis; rumenitis; Balantidium coli y acidosis ruminal
INTRODUCTION
There are many diseases or digestive system disorders in cattle. They are of economic importance in the production units, since they cause a decrease in production, high morbidity and mortality; they reduce animal welfare, as well as an impact on public health (Plaizier et al., 2008). The rumen carries out a series of adaptations to the different feeding phases, to which the bovines are subjected, in which forages and large quantities of grains are digested; which can cause the appearance of digestive disorders, such as acidosis, resulting from the rapid fermentation of the grain (Meyer y Bryant, 2017). Ruminal acidosis (RA) problems are common. However, it is a difficult disorder to recognize and prevent, since the pathophysiology is complex and variable (Oetzel, 2017). This disease is caused by eating high concentrate diets; it is defined as a decrease in pH (<5.6), for prolonged periods of up to at least three hours per day (Kleen et al., 2003; Gozho et al., 2005; Krause y Oetzel, 2006; Steele et al., 2009).
Low pH induces lysis of Gram-negative bacteria, and increases free lipopolysaccharide (LPS) in the rumen, which is a powerful inducer of inflammation and the development of rumenitis (Zhao et al., 2018). The production of immunogens in the rumen, such as LPS or histamine, cause the rumen protection barrier to be reduced and they are implicated in the development of this disease (Plaizier et al., 2008).
Ruminal pH is a key factor for the correct functioning of the rumen, since it affects microbial populations, products of fermentation and its physiological functions (Nagaraja y Titgemeyer, 2007). A decrease in: fat in milk, reduced fiber digestion, loss of body condition, diarrhea, laminitis, inflammation and tympanism can be observed; in addition to affecting the voluntary consumption of food (Panciera et al., 2007; Rezac et al., 2014a; Zhao et al., 2018).
These alterations can cause an imbalance in the ruminal microbiota, where etiological agents, such as Fusobacterium necrophorum, Trueperella pyogenes and Arcanobacterium spp, among others, behave like opportunists, and travel through the portal circulation to the liver, where they can generate abscesses (Tadepalli et al., 2009; Xu y Ding, 2011; Trigo, 2015). Alteration of the ruminal epithelium allows microorganisms and their toxins to migrate into the portal circulation, which can predispose to a variety of infections or endotoxins in the bloodstream, as they alter the ability of nutrient absorption; which can generate an accumulation of volatile fatty acids (AGV) or lactic acid (Kleen et al., 2003). The ruminal papillae are usually by mucus compared to the abomasal papillae not covered, so they may be more susceptible to rumenitis that can progress to erosion and ulceration (Snyder y Credille, 2017).
Parakeratosis is the thickening of the stratum corneum of the ruminal epithelium, and it is the result of chronic rumenitis (Jubb et al., 2016), being an important pathology; since it decreases the absorption capacity of AGV and predisposes affected animals to new episodes (Oetzel, 2017). In Mexico, the economic impact produced by ruminal acidosis has been difficult to quantify, but it is important due to the injuries it causes in fattening cattle (Malafaia et al., 2016).
There are few studies in Mexico that describe the injuries observed in the rumen at the time of the slaughter of the animals, the rumen inspection is a tool that allows evaluating the nutritional health of livestock in the pens. Therefore, the objective of the present study was to describe the ruminal lesions present in cattle that arrive at the municipal meat processor in Colima city, Mexico.
MATERIAL AND METHODS
Study area
The work was in Colima state carried out, at the municipal meat processor, located at km.3.5 of the Colima-Coquimatlán highway. The predominant climate in 78.52% of the territory is warm sub-humid with rains in summer, in 12.42% there is a semi-dry warm climate, in 7.58% semi-warm sub-humid with rains in summer and in 1.48% of the territory, a temperate sub-humid climate with rains in summer (INEGI, 2017). The average annual temperature is 25 °C and the total annual precipitation is approximately 1728.9 mm (CONAGUA, 2017).
Sampling
100 samples of ruminal tissue were collected, 1 cm2 from the right side of the cranio- ventral sac and from the left side of the cranial ventral sac, because in this ruminal portion the epithelium is in longer contact with ruminal fluid (Castro y Elizondo, 2012; Jonsson et al., 2019). Some representative areas, where some type of macroscopic injury has been observed; which were fixed by immersion in 10% buffered formalin for 24 h. The tissues were in plastic cassettes, cut and included. They were transferred to the Pathology Laboratory of the Faculty of Veterinary Medicine and Animal Husbandry of the University of Colima, located in Tecomán municipality, Colima, Mexico at km. 40, Colima-Manzanillo highway, within the geographic coordinates 18° 55’ of North Latitude and 103° 52’ of West Longitude, and an altitude of 20 m a.s.l (INEGI, 2017). Subsequently, they were processed with the routine histological technique, which consists of dehydration, clearance and paraffin infiltration; they were cut at 5 µm thick and stained with Hematoxylin-Eosin (H-E) staining (Prophet et al., 1995).
Sample analysis
Macroscopic and microscopic lesions were using pathological concepts described and characterized; the results were expressed in percentages and degrees of severity (Rezac et al., 2014b) Likewise, the Chi square test was used to assess the dependency between the lesions with the place of origin of the animals, age, species and sex. A logistic regression analysis was performed in order to find risk factors associated with the presence of injuries. Both analyzes were performed with the Statgraphics Centurion ver.15.2 program (Statgraphics, 2007).
For the statistical analysis, two age ranges were established, less than or equal to 36 months, and greater than or equal to 37 months; trying to maintain an adequate number of records at each level of classification. For the species, the Angus, Beefmaster, Brown Swiss and Simmental racial genotypes were classified in Bos Taurus and zebu were classified in Bos indicus. The places of origin were into four regions grouped, according to the type of climate: region 1: temperate sub-humid, region 2: semi-warm sub-humid, region 3: warm sub-humid and region 4: warm.
RESULTS
From the total samples, 33 were females and 67 males. Of the slaughtered animals, 56 belonged to the Bos taurus species and 44 to Bos indicus. The 98% of the samples presented at least one lesion. The macroscopic findings were (figure 1): hemorrhages, erosion, ulcers, hyperkeratosis (thickening of the rumen papillae), atrophy of the papillae and scars; one sample had a papilloma. The microscopic findings (figure 2) were thickening of the horny layer of the ruminal papillae, as well as the presence of collagen fibers, hydropic degeneration, inflammation (rumenitis) and lymphangiectasia. In addition, the presence of the protozoan Balantidium coli was recorded in 30% of the samples.

Figure 1 Macroscopic description of ruminal lesions. A/ normal rumen mucosa. B/ thickening and atrophy of the rumen papillae. C/ ruminal hemorrhages. D/E erosions, ulcers and ruminal scars. F/ presence of 1X2 cm wart or papilloma showing a cauliflower-like mass, white in color, growing above the epithelium on the surface of the ruminal mucosa and covered with scales.
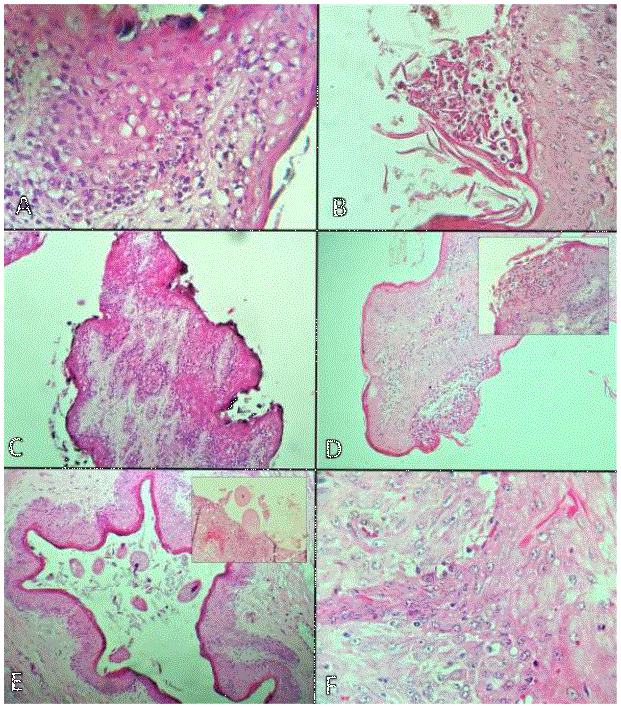
Figure 2 Microscopic description of ruminal lesions. A/ Thickening of the horny layer of the ruminal papillae, as well as the presence of epithelial hydropic degeneration. Hematoxylin-eosin 40X staining. B-C/ Erosion, ulcer and parakeratosis of the ruminal papilla. Hematoxylin-eosin 40X staining. D/ Mononuclear inflammatory infiltrate over the submucosa of the ruminal papilla (rumenitis), inset with zoom. Hematoxylin- eosin 40X staining. E/Presence of abundant protozoa Balantidium coli on the mucosa and ruminal epithelium causing hemorrhages and inflammation (box). Hematoxylin-eosin staining 10-40X. F/Epithelial hyperplasia of the ruminal papillae with cytoplasmic vacuolization and hyperkeratosis, in addition to proliferation of collagen, forming digital projections towards the submucosa (papilloma). Hematoxylin-eosin 40X staining.
These injuries were grouped into degrees of severity, which ranged from mild to severe (Table 1).
Table 1 Number of cases for rumen injuries and their classification in degrees severity
| Degree of severity | Hyperkeratosis | Hydropic degeneration | Rumenitis | Lymphagiectasia | Balantidiumcoli |
|---|---|---|---|---|---|
| Slight | 33 | 53 | 23 | 21 | 20 |
| Moderate | 42 | 30 | 18 | 6 | 6 |
| Severe | 18 | 9 | 1 | 1 | 4 |
| Total case | 93 | 92 | 42 | 28 | 30 |
| No apparent injury | 7 | 8 | 58 | 72 | 70 |
According to the frequencies found, bovines from sub-humid semi-warm climates, whose species was Bos indicus, older than or equal to 37 months and males, presented more injuries. The presence of the protozoan B. coli was significant in the Chi square test, showing dependence with the origin (P=0.046) and sex (P=0.041) of the animals. In the logistic regression analysis the variables: origin, age, species and sex of the animals, were significant (P=0.000) for the presence of scars, rumenitis, lymphangiectasia and B. coli (Table 2).
Table 2 Risk factors for the presence of ruminal lesions in cattle slaughtered at the Municipal Meat Processor in Colima. Mexico
| IC(OR)95% | |||||||
|---|---|---|---|---|---|---|---|
| Variables | Estimate | Stándar error | Odd Ratio | Superir limit (LI) | Inferior limit (LS) | Value P | |
| Cicatriz | |||||||
| Region 1 | -0.230 | 0.067 | 0.793 | 0.694 | 0.907 | ||
| Provenance | Region 2 | -0.685 | 0.05 | 0.503 | 0.455 | 0.556 | 0.000 |
| Region 3 | 0.402 | 0.089 | 1.495 | 1.250 | 1.787 | ||
| Age | -0.142 | 0.045 | 0.866 | 0.791 | 0.949 | 0.000 | |
| Species | -0.515 | 0.046 | 0.597 | 0.545 | 0.654 | 0.000 | |
| Sex | -0.299 | 0.049 | 0.741 | 0.672 | 0.817 | 0.000 | |
| Rumenitis | |||||||
| Region 1 | 0.949 | 0.0711 | 2.583 | 2.243 | 2.975 | ||
| Provenance | Region 2 | 0.176 | 0.049 | 1.192 | 1.080 | 1.317 | 0.000 |
| Region 3 | -1.001 | 0.080 | 0.367 | 0.313 | 0.430 | ||
| Age | 0.310 | 0.045 | 1.364 | 1.246 | 1.494 | 0.000 | |
| Species | 0.725 | 0.044 | 2.066 | 1.891 | 2.258 | 0.000 | |
| Sex | -0.092 | 0.048 | 0.911 | 0.828 | 1.003 | 0.000 | |
| Lymphagiectasia | |||||||
| Region 1 | -1.177 | 0.067 | 0.308 | 0.269 | 0.352 | ||
| Provenance | Region 2 | -0.066 | 0.056 | 0.935 | 0.836 | 1.046 | 0.000 |
| Region 3 | -0.431 | 0.083 | 0.649 | 0.55 | 0.765 | ||
| Age | -0.448 | 0.049 | 0.638 | 0.578 | 0.704 | 0.000 | |
| Species | -0.266 | 0.05 | 0.766 | 0.693 | 0.846 | 0.000 | |
| Sex | 0.453 | 0.056 | 1.573 | 1.406 | 1.759 | 0.000 | |
| Balantidium coli | |||||||
| Region 1 | -1.211 | 0.074 | 0.297 | 0.256 | 0.344 | ||
| Provenance | Region 2 | -1.229 | 0.06 | 0.292 | 0.259 | 0.329 | 0.000 |
| Region 3 | -1.657 | 0.085 | 0.19 | 0.16 | 0.225 | ||
| Age | -0.477 | 0.05 | 0.62 | 0.56 | 0.686 | 0.000 | |
| Species | 0.501 | 0.048 | 1.65 | 1.499 | 1.816 | 0.000 | |
| Sex | -0.779 | 0.052 | 0.458 | 0.413 | 0.5 | 0.000 | |
(P<0.05)
4 was, as a comparison for the provenance variable taken: warm climate; for the age the bovines smaller than 36 months for the sex the females and for the Bos taurus species.
Cattle from semi-humid sub-humid climates were 1.49 times more likely to present rumen scars; compared to animals from warm climates. Cattle from subhumid temperate climates were 2.5 times more likely to present rumenitis than the ones from warm climates. Cattle older than 37 months were 1.3 times more likely to present rumenitis, compared to cattle less than or equal to 36 months. The Bos taurus species was 2.06 times more likely to have rumenitis, compared to Bos indicus. Similarly, Bos taurus was 1.65 times more likely to present B. coli than the Bos indicus species. Male cattle were 1.57 times more likely to have lymphangiectasia, compared to females. The Bos indicus species was 1.65 times more likely to present B. coli than the Bos taurus species.
DISCUSSION
The bovines used for meat production in Colima state, come from prairie or mixed fattening systems. In mixed systems, the initiation stage begins in the meadow and ends in the pens, with the challenge of fattening for approximately 100-120 days, with the known “hot diets” consisting of the administration of large amounts of concentrate and the consequent decrease in rumen pH. It causes the appearance of rumen lesions; figure that in the present study was higher than those found by Rezac et al. (2014a).
In a study carried out in the United States on serious pathological conditions in cattle at slaughter, ruminal lesions had a higher frequency (35%) in 1461 cattle, the rumenitis symptoms with a decrease in the papillae was the most common lesion. The presence of scars and areas without papillae indicates a long-term exposure of the epithelium to an unfavorable environment, which may explain the degree of severity of the lesions and their frequency in adult animals. Steele et al. (2009) observed a detachment of the stratum corneum from the ruminal papillae, after submitting a bovine to a high grain diet, an injury not observed in the present study. It coincides with Sasikala et al. (2018), where the in vivo rumen content was observed a cornified epithelium with changes of coloration and necrosis by endoscopy in 110 cattle, which detached easily showing a hemorrhagic surface. Biopsy revealed vacuolar degeneration with nuclear changes of the squamous epithelium. Diet is the main factor in bacterial structure and ruminal function, since the epithelial bacteria of the rumen bind to the epithelial tissue, so the alteration in its composition can affect function and generate lesions (Zhang et al., 2017).
Studies suggest that there is an increase in the presence of bacteria with high virulence genes, which can take advantage of these ruminal conditions to trigger an inflammatory response (Khafipour et al., 2011) or modify genes involved in the growth and structure of epithelia (Steele et al., 2011). The evaluation of the ruminal microbiota in the present study was not considered, but it is believed that the bacterial diversity of the rumen and its response to diet varies considerably; even among animals fed the same diet (Penner et al., 2009; Chen et al., 2011).
In the present study, it was observed that the Bos taurus species showed a greater tendency to present rumen lesions, compared to the Bos indicus species; the above, according to Lees et al. (2017) it was mentioned that the rumen adaptation process is different between animals of the Bos taurus and Bos indicus species, particularly in tropical and subtropical climates, where zebuines have a better ability to adapt to tropical climates with temperature and high humidity, compared to Bos taurus (Reis et al., 2016). Panciera et al. (2007) registered severe damages, due to acidosis, such as: abomasal tympanism, marked edema, hemorrhage and emphysema in the rumen walls; where it is suggested that part of the damage was due to the presence of clostridial species, or a group of agents that are involved in these damages. The presence of foreign objects in the rumen, such as plastic bags, can cause damage to the epithelium, such as atrophy, loss of rumen papillae, erosion, ulcers and nodular formation in the rumen mucosa (Otsyina et al., 2017). The presence of ciliated protozoa is associated with the structure and pathogenicity of different bacterial communities and alterations in ruminal fermentation (García et al., 2017). In addition of being an opportunistic pathogen, B. coli is zoonotic, and it causes gastroenteric and lung problems in man (Sharma y Harding, 2003; Pérez et al., 2008; Koopowitz et al., 2010).
The temperature and humidity conditions of Colima state allow the viability of this protozoan in the environment, and the infection occurs through the consumption of water or food contaminated with oocytes, excreted by animals, or even infected humans (Ahmed et al., 2020).
In a study conducted in Bangladesh by Paul et al. (2019), identified the presence of the protozoan parasite B. coli in 103/200 fecal samples. The highest prevalence was recorded in cattle (54.7%), compared to pigs (42%), a result higher than that found in the present study. In China, Zhang et al. (2019), carried out an analysis in 468 fecal samples to identify the main infectious agents found in macaques, B. coli, was the second most important protozoan with 70% prevalence, a result higher than that of other species. In Brazil, a study in birds in captivity showed a prevalence of 1.4% for this same parasite using the coproparasitoscopic technique, which was found in pigeons, suggesting that they may be reservoirs for other susceptible hosts (Lyra et al., 2002). Acidosis is a feeding management problem. It occurs when cattle that were consuming grass, quickly change to a high carbohydrate diet, as in most fattening; in which the addition of some buffer to the ration such as sodium bicarbonate or calcium carbonate in no more than 5% of the ration. It makes gradual changes in the transitions of the fattening stage, as well as make routine inspections of the digestive system at the time of slaughter.
CONCLUSIONS
Carrying out this study allowed us to identify the main types of ruminal lesions in cattle that come to slaughter to the Colima municipal meat processor. Almost the totality of bovine animals sampled suffered some type of acidosis during their life, which caused lesions in the rumen wall, causing economic losses; due to the low yields in the fattening pens. Post mortem inspection in bovines is a tool that allows evaluating ruminal health, as well as promoting preventive measures for the control of subclinical ruminal acidosis. The presence of unusual agents in the rumen suggests an alteration in the microbiota of the ruminal epithelium. This is the first study where the presence of Balantidium coli in ruminal tissues is documented.
LITERATURA CITADA
Ahmed A, Ljaz M, Ayyub RM, Ghaffar A, Ghauri HN, Aziz MU, Ali S, Altaf M, Awais M, Naveed M, Nawab Y, Javed MU. 2020. Balantidium coli in domestic animals: an emerging protozoan pathogen of zoonotic significance. Acta Trópica. 203(1)1-12. https://doi.org/10.1016/j.actatropica.2019.105298 [ Links ]
Castro FP, Elizondo SJA. 2012. Crecimiento y desarrollo ruminal en terneros alimentados con iniciador sometido a diferentes procesos. Agronomía Mesoamericana. 23(2):343-352. ISSN: 1021-7444 https://www.redalyc.org/articulo.oa?id=43724664013 [ Links ]
Chen Y, Penner GB, Li M, Oba M, Guan LL. 2011. Changes in bacterial diversity associated with epithelial tissue in the beef cow rumen during the transition to a high-grain diet. Applied and Environmental Microbiology. 77(16):5770-5781. ISSN:1098-5336. https://doi.org/10.1128/AEM.00375-11 [ Links ]
CONAGUA (COMISIÓN NACIONAL DEL AGUA). 2017. Sistema meteorológico nacional, resúmenes mensuales y anuales de temperaturas y lluvia. Disponible en la Web: http://smn.cna.gob.mx/es/climatologia/temperaturas-y-lluvias/resumenes-mensuales-de- temperaturas-y-lluvias [ Links ]
García M, Bradford BJ, Nagaraja TG. 2017. Invited Review: Ruminal microbes, microbial products and systemic inflammation. The professional Animal Scientist. 33(6):635-650. ISSN:1080-7446. https://doi.org/10.15232/pas.2017-01663 [ Links ]
Gozho GN, Plaizier JC, Krause DO, Kennedy AD, Wittenberg KM. 2005. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. Journal of Dairy Science. 88(4):1399-1403. ISSN:1525-3198. https://doi.org/10.3168/jds.S0022-0302(05)72807-1 [ Links ]
INEGI (INSTITUTO NACIONAL DE ESTADÍSTICA Y GEOGRAFÍA). 2017. Anuario estadístico y geográfico de Colima 2017. Disponible en la Web: http://internet.contenidos.inegi.org.mx/contenidos/Productos/prod_serv/contenidos/espa nol/bvinegi/productos/nueva_estruc/anuarios_2017/702825092061.pdf [ Links ]
Jonsson NN, Ferguson HJ, Koh-Tan HH, McCartney CA. 2019. Postmortem observations on rumen wall histology and gene expression and ruminal and caecal content of beef cattle fattened on barley-based rations. Animal. 1-14. https://doi.org/10.1017/S1751731119002878 [ Links ]
Jubb KV, Kennedy PC, Palmer NC. 2016. Pathology of domestic animals 6th edition. Vol. 2. Elsevier St. Louis Missouri. USA. Pp: 39-42. ISBN 9780702053184 [ Links ]
Khafipour E, Plaizier JC, Aikman PC, Krause DO. 2011. Population structure of rumen Escherichia coli associated with subacute ruminal acidosis (SARA) in dairy cattle. Journal of Dairy Science. 94(1):351-360. ISSN:1525-3198. https://doi.org/10.3168/jds.2010-3435 [ Links ]
Kleen JL, Hooijer GA, Rehage J, Noordhuizen JP. 2003. Subacute ruminal acidosis (SARA): A review. Journal Veterinary Medicine a Physiology Pathologic Clinic Medicine 50(8):406-414. ISSN:1439-0442. https://doi.org/10.1046/j.1439-0442.2003.00569.x [ Links ]
Koopowitz A, Smith P, Rensburg NV, Rudman A. 2010. Balantidium coli induced pulmonary hemorrhage with iron deficiency. South African Medical Association. 100(8):534-536. ISSN:2078-5135. http://www.samj.org.za/index.php/samj/article/view/3592 [ Links ]
Krause KM, Oetzel RG. 2006. Understanding and preventing subacute ruminal acidosis in dairy herds: A review. Animal Feed Science and Technology. 126(3-4):215-236. ISSN:0377-8401.https://doi.org/10.1016/j.anifeedsci.2005.08.004 [ Links ]
Lees AM, Lees JC, Lisle AT, Sullivan ML, Gaughan JB. 2017. Effect of heat stress on rumen temperature of three breeds of cattle. International Journal of Biometeorology. 62(1):207-215. https://doi.org/10.1007/s00484-017-1442-x [ Links ]
Lyra MF, Olivera JB, Brito CMD, Soares LA, Magalhares VS, Oliveira RA, Sobrino AE. 2002. Parásitos gastrointestinales de aves silvestres en cautiverio en el estado de Pernambuco, Brasil. Parasitología Latinoamericana. 57(1-2):50-54. ISSN:0717-7712. http://dx.doi.org/10.4067/S0717-77122002000100012 [ Links ]
Malafaia P, Lima GTA, Costa RM, Carneiro SV, Azevedo CDF, Tokarnia CH. 2016. Major health problems and their economic impact on beef cattle under two different feedlots systems in Brazil. Pesquisa Veterinária Brasileira. 36(9):837-843. ISSN:1678- 5150. https://doi.org/10.1590/s0100-736x2016000900008 [ Links ]
Meyer NF, Bryant TC. 2017. Diagnosis and management of rumen acidosis and bloat in feedlots. 33(3):481-498. Veterinary Clinics of North America: Food Animal Practice. ISSN:1558-4240. https://doi.org/10.1016/j.cvfa.2017.06.005 [ Links ]
Nagaraja TG, Titgemeyer EC. 2007. Ruminal acidosis in beef cattle: the current microbiological and nutritional outlook. Journal of Dairy Science. 90(1):E17-E38. ISSN:1525-3198. https://doi.org/10.3168/jds.2006-478 [ Links ]
Oetzel GR. 2017. Diagnosis and management of subacute ruminal acidosis in dairy herds. Veterinary Clinics of North America. Food Animal Practice. 33(3):463-480. ISSN:1558-4240. https://doi.org/10.1016/j.cvfa.2017.06.004 [ Links ]
Otsyina HR, Mbuthia PG, Nguhiu-Mwangi J, Mogoa EG, Ogara WO. 2017. Gross and histopathologic findings in sheep with plastic bags in the rumen. International Journal of Veterinary Science and Medicine. 5(2):152-158. ISSN:2314-4599. https://doi.org/10.1016/j.ijvsm.2017.08.005 [ Links ]
Panciera RJ, Boileau JM, Step LD. 2007. Tympany, acidosis, and mural emphysema of the stomach in calves: report of cases and experimental induction. Journal of Veterinary Diagnostic Investigation. 19(4):392-395. ISSN:1040-6387. https://doi.org/10.1177/104063870701900409 [ Links ]
Paul TR, Begum N, Shahiduzzaman M, Hossain MS, Labony S, Anisuzzaman ARD. 2019. Balantidiasis, a zoonotic protozoan infection, in cattle and domestic pigs. Bangladesh Journal of Veterinary Medicine. 17(1):31-37. ISSN:2308-0922. https://doi.org/10.33109/bjvmjj19fam1 [ Links ]
Penner GB, Aschenbach JR, Gäbel G, Rackwitz R, Oba M. 2009. Epithelial capacity for apical uptake of short chain fatty acids is a key determinant for intraruminal pH and the susceptibility to subacute ruminal acidosis in sheep. The Journal of Nutrition. 139(9):1714- 1720. ISSN:1541-6100. https://doi.org/10.3945/jn.109.108506 [ Links ]
Pérez-Cordón G, Rosales MJ, Valdez RA, Vargas-Vásquez F, Cordova O. 2008. Detección de parásitos intestinales en agua y alimentos de Trujillo, Perú. Revista Peruana de Medicina Experimental y Salud Pública. 25(1):144-148. ISSN:1726-4634. http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1726-46342008000100018 [ Links ]
Plaizier JC, Krause DO, Gozho GN, Mcbride BW. 2008. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. The Veterinary Journal. 176(1):21-31. ISSN:1532-2971. https://doi.org/10.1016/j.tvjl.2007.12.016 [ Links ]
Prophet EB, Mills B, Arrington JB, Sobin LH. 1995. Métodos Histotecnológicos. Instituto de Patología de las Fuerzas Armadas de los Estados Unidos de América (AFIP). Washington, DC: Publicado por el registro de Patología de los Estados Unidos de América (ARP). ISBN:1-881041-21-2. [ Links ]
Reis AS, Bomjardim HA, Oliveira CM, Oliveira CH, Silveira JA, Silva ND, Salvarani FM, Silva JB, Barbosa JD. 2016. Vagal indigestion in Zebu cattle in Brazil. Revista Salud Animal. 38(3):149-153. ISSN: 2224-4700 http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0253- 570X2016000300003&lng=es&nrm=iso&tlng=en [ Links ]
Rezac DJ, Thomson DU, Siemens MG, Prouty FL, Reinhardt CD, Bartle SJ. 2014a. A survey of gross pathologic conditions in cull cows at slaughter in the Great Lakes region of the United States. Journal of Dairy Science. 97(7):1-9. ISSN:1525-3198. https://doi.org/10.3168/jds.2013-7636 [ Links ]
Rezac DJ, Thomson DU, Bartle SJ, Osterstock JB, Prouty FL, Reinhardt CD. 2014b. Prevalence, severity and relationships of lung lesions, liver abnormalities, and rumen health scores measured at slaughter in beef cattle. Journal of Animal Science. 92(6):2595-2602. ISSN:1525-3163. https://doi.org/10.2527/jas.2013-7222 [ Links ]
Sasikala K, Vijayakumar G, Sivaraman S, Balasubramaniam GA. 2018. Ruminoscopy in Cattle (Bos Taurus) with Ruminal Lactacidosis-A rapid and novel Method to visualize rumen papillary changes. International Journal of Current Microbiology and Applied Sciences. 7(5):3112-3119. ISSN:2319-7706. https://doi.org/10.20546/ijcmas.2018.705.363 [ Links ]
Sharma S, Harding G. 2003. Necrotizing lung infection caused by the protozoan Balantidium coli. The Canadian Journal of Infectious Diseases. 14(3):163-166. ISSN:1180-2332. https://doi.org/10.1155/2003/829860 [ Links ]
Snyder E, Credille B. 2017. Diagnosis and treatment of clinical rumen acidosis. Veterinary Clinics of North America: Food Animal Practice. 33(3):451-461. ISSN:0749-0720. https://doi.-org/10.1016/j.cvfa.2017.06.003 [ Links ]
Statgraphics® Centurion XV. 2007. User Manual. Version 15.2.06. StatPoint Technologies, Inc. http://www.statgraphics.com [ Links ]
Steele MA, Croom J, Kahler M, AlZahal O, Hook SE, Plaizier K, McBride BW. 2011. Bovine rumen epithelium undergoes rapid structural adaptations during grain-induced subacute ruminal acidosis. American Journal of Physiology. Regulatory, Integrative and Comparativ Physiology. 300(6):1515-1523. ISSN:1522-1490. https://doi.org/10.1152/ajpregu.00120.2010 [ Links ]
Steele MA, AlZahal O, Hook SE, Croom J, McBride BW. 2009. Ruminal acidosis and the rapid onset of ruminal parakeratosis in a mature dairy cow: a case report. Acta Veterinaria Scandinavica. 51(39):1-6. ISSN: 1751-0147. https://doi.org/10.1186/1751-0147-51-39 [ Links ]
Tadepalli S, Narayanan SK, Stewart GC, Chengappa MM, Nagaraja TG. 2009. Fusobacterium necrophorum: A ruminal bacterium that invades liver to cause abscesses in cattle. Anaerobe. 15(1-2):36-43. ISSN:1075-9964. https://doi.org/10.1016/j.anaerobe.2008.05.005 [ Links ]
Trigo TF. 2015. Patología Sistémica Veterinaria. 6ta edición. México D.F. Pp. 140-142. ISBN: 9786070278099 [ Links ]
Xu Y, Ding Z. 2011. Physiological, biochemical and histopathological effects of fermentative acidosis in ruminal production: a minimal review. Spanish Journal of Agricultural Research9(2):414-422. ISSN:1695-971-X. http://dx.doi.org/10.5424/sjar/20110902-177-10 [ Links ]
Zhang Q, Han S, Liu K, Luo J, Lu J, He H. 2019. Ocurrence of selected zoonotic fecal pathogens and first molecular identification of hafnia paralvei in wild Taihangshan Macaques (Macaca mulatta tcheliensis) in China. Hindawi BioMed Research International. 2019(1):1-7. ISSN:2314-6141. https://doi.org/10.1155/2019/2494913 [ Links ]
Zhang R, Ye H, Liu J, Mao S. 2017. High-grain diets altered rumen fermentation and epithelial bacterial community and resulted in rumen epithelial injuries of goats. Applied Microbiology and Biotechnology. 101(18):6981-6992. ISSN:1432-0614. https://doi.org/10.1007/s00253-017-8427-x [ Links ]
Zhao C, Liu G, Li X, Guan Y, Wang Y, Yuan X, Sun G, Wang Z, Li X. 2018. Inflammatory mechanism of rumenitis in dairy cows with subacute ruminal acidosis. BMC Veterinary Research. 14(135):3-8. ISSN: 1746-6148. https://doi.org/10.1186/s12917-018-1463-7 [ Links ]
Received: January 05, 2020; Accepted: May 20, 2020











 texto en
texto en 


