INTRODUCTION
Methane (CH4), produced by domestic ruminants, represents a loss of 2 to 12% of gross energy (EB) intake (Hristov et al., 2015, Moumen et al., 2016), significant inefficiency in the systems of production of these (Moss et al., 2000, Moumen et al., 2016). One mole of CH4, has the same EB as to remove acetate; that is, 0.88 MJ mol-1, thus the net metabolizable energy of any diet increases the reducing power of the CH4 formation in the final products that are usable by the ruminant. An adult sheep consumes 22 Kg dry matter (DM), with average gross energy (EB) loss of 6%, and produces about 27 mol CH4, which equals 2.5 kg of fermented glucose to 27 mol of acetate (Johnson et al. ., 1991, Moss et al., 2000).
Fermentation and ruminal degradation is mainly the result of the activity of bacteria, protozoa and fungi that inhabit the rumen of the animal, which act on the different fractions that make up the food. In particular, this ruminal microflora makes it possible that the ingested fibrous fraction that cannot be enzymatically digested by the animal, is degraded and fermented towards volatile fatty acids, fermentation gases and heat; or incorporated into digestible microbial matter (Alejos et al., 2018).
Galicia et al. (2017), point out that the ability to adapt quickly to changes in the environment is one of the main characteristics of the bacterial cell; the rumen is a highly dynamic environment, and none of the changes is permanent, due to the various microbial species found in the rumen. Signal transduction networks are the information processing pathways that recognize various physical and chemical stimuli, amplification, and signal processing that trigger bacterial cell adaptation responses.
Getachew et al. (2005) and Tapio (2017), mention that the microbial fermentation that occurs in the rumen, generates products that can be used by the animal; such as volatile fatty acids, microbial protein and other types of products that are considered waste, such as carbon dioxide and methane. The microbial fermentation of the fiber comprises several sequential actions, which are pointed out by France (2005) and these are: hydration, adherence of the appropriate microorganisms, release of a mixture of hydrolytic enzymes and finally hydrolysis. The resulting release of monomers is followed by their subsequent intracellular degradation in volatile fatty acids (VFA) and fermentation gases.
It should be considered that, along with carbohydrates, the fermentation of the lipids and protein of the ration also contribute to the production of volatile fatty acids; being lower for lipids and considerable for proteins when diets have high content of degradable protein in the rumen (Popova et al., 2017). For the microbial population, most of these VFAs are waste products; but for the ruminant they represent the main source of absorbed energy, which corresponds to 50 to 70% of the digestible energy (Zhang, 2017). It is reported that 6 to 10% of the gross energy consumed by a cow is converted to methane, and it is eliminated by the respiratory route (Eckard et al., 2010). The excess of dihydrogen (H2) generated during the conversion of glucose to acetic or butyric acid, and the CO2 produced by the decarboxylation of metabolites during fermentation, are used by methanogenic archaea; which use them as substrates to form methane (Alayón et al., 2018). It is important to highlight the relevance of protozoa in methane formation processes, since they maintain a symbiotic relationship with methanogenic archaea, with those who are intra- and extra-cellularly associated and carry out the transfer of this hydrogen (Hook et al., 2010).
There are methodologies used to reduce the amount of CH4 produced by ruminants; among them the implementation of chemical additives in livestock feed; however, the use of these has been limited by the residual appearance in products for human consumption, which led to its prohibition in some legislations, such as that of the European Union in January 2006 (Alejos et al., 2018). These measures gave rise to the search for new strategies and alternatives oriented to the use of natural additives, such as plant extracts; which are an option in livestock feeding to modify their ruminal fermentation in a positive way and mitigate the enteric CH4 emissions (Wallace, 2004).
Therefore, practices oriented to methane mitigation can be important factors that impact the profitability of a production system (Alayón et al., 2018). In this sense, it is important to consider that any mitigation practice that requires additional investment and that is not compensated by external agents, or that affects animal productivity, or increases production costs; it is very likely that it has been rejected by the producer (Gerber et al., 2013).
A mitigation alternative to avoid increases in methane production is the use of plant extracts; which contain bioactive compounds that have been investigated as an alternative in animal nutrition, changing the degradability and fermentation of the food; this effect is related to its chemical activity and/or antimicrobial properties of the extracts (Patra and Saxena, 2010). Therefore, the objective was to obtain and characterize plant extracts (Larrea tridentata, Origanum vulgare, Artemisa ludoviciana and Ruta graveolens), to test them in ruminal digestibility in vitro and quantify gas production, AGV and methane production.
MATERIAL AND METHODS
Region of study and collection of samples
The study was carried out in Zacatecas state, Mexico, using plants (leaves and flower) of Larrea tridentata, Origanum vulgare, Artemisa ludoviciana and Ruta graveolens; these were collected from wild growth during spring-summer of 2014 and 2015 at random. Each of the plants was dried at room temperature for two weeks, then dried at 45 °C in a conventional oven for 24 h to remove moisture; they were ground and stored in plastic bags.
Obtaining plant extracts
In the extraction, 20 g of the ground sample was used for each 300 ml of ethanol, 70% (J.T. Baker); it was placed in amber bottles. The mixture was sealed and stirred vigorously for 10 min, allowed to soak for one month at room temperature. The supernatant was filtered (Whatman No. 2), to remove the remains of the powder from the plant and the solvent was evaporated in a Soxhlet type extractor at 85 °C. Two aliquots were made; one was used to determine the chemical composition of the extract and the other was used to evaluate the antimicrobial activity.
Chemical composition of the extract by gas chromatography
It was determined by means of a gas chromatograph (CG, Agilent Technologies 6890N series), using the polar column DB_WAXetr. The working conditions were: temperature after injection 250 °C, at a pressure of 12.13 psi with a flow of He 36.5 ml min-1. The conditions for the column were: initial temperature 50 °C, from zero to two minutes, with an increase of 10 °C until reaching 250 °C; maintaining this temperature constant for 5 min, then descending to 50 °C for two minutes, with a He flow of 1.6 mL min-1 at a pressure of 12.13 psi and an average velocity of 25 cm s-1. Using an ionizing flame detector (FID) at a temperature of 210 °C, with a flow of H2 of 40 ml min-1 and a flow of air of 450 ml min-1. Previously a calibration curve was performed, the standards used were: reactive grade, Sigma Aldrich brand: carvacrol, thymol, limonene, linalool and terpinene, with a purity percentage of 98, 99.5, 98, 97 and 85% respectively. Each of the determinations was made in triplicate.
Determination of in vitro gas production
Feeding the sheep
For in vitro gas production, ruminal fluid from two hair sheep, cannulated, fed with a diet containing 83% hay (50% alfalfa and 50% wheat straw) and 17% concentrate (63%) ground corn, 25% flourolin, 5.5% calcium carbonate, 5.5% mono-calcium phosphate, 0.5% pre-mixed vitamins A, D and E, 0.5% microminals) (NRC, 2003) was used. The food was provided daily at 08:00 and 16:00 hours, with free access to water. The sheep were fed for 30 days before the extraction of the ruminal fluid, as adaptation time to the ration.
In vitro gas production
The extracts were added individually in each of the digestibility jars, in different volumes (Agarwal et al., 2009); as a control the alfalfa substrate was used without the addition of additives. In vitro gas production was determined using the method proposed by Theodorou et al. (1994); for which fermentation units (UF) of 120 ml were used for each sample. In the gas register produced, a Sper Scientific brand pressure meter was used; the gas pressure was cumulative and determined in units of pressure (Psi); the measurement time was at 3, 6, 9, 12, 24 and 48 h. For each volume of the different extracts, three repetitions were made.
Determination of volatile fatty acids in ruminal fluid
The AGVs (acetic, propionic and butyric) were quantified by gas chromatography. The working conditions were: inlet temperature after injection of the sample is 50 °C at a pressure of 12.13 psi, with a flow of He 36.5 ml min-1. The conditions for the column were: initial temperature 50 °C, from zero to two minutes with an increase of 10 °C per minute until reaching 250 °C, keeping this temperature constant for 5 minutes, then descending to 50 °C maintaining for two minutes with a He flow of 1.6 ml min-1 at a pressure of 12.13 psi and an average velocity of 25 cm s-1.
An ionizing flame detector (FID) was used at a temperature of 210 ° C, with a flow of H2 of 40 ml min-1 and an air flow of 450 ml min-1. Previously a calibration curve was made. The standards used were: reactive grade, Sigma Aldrich brand: acetic, propionic and butyric; with a purity percentage of 99.5, 98 and 99 respectively. Each of the determinations was made in triplicate
Determination of Methane
Methane was inferred from the concentration of AGV, through the application of nonlinear mathematical models established by Moss et al. (2000); where it is indicated that the production of CH4 can be calculated stoichiometrically, using the following equation:
RESULTS AND DISCUSSION
The yield results of the extracts L. tridentata, O. vulgaris, A. ludiviciana and R. graveolans (Table 1), are due to the time of concentration of the sample in the Soxleth equipment, obtaining the volume of the extract (ml), equivalent to the percentage yield (w/w), this depends on the time and mode of the process, from its collection until its extraction. The yields of extracts one and two of L. tridentata were 11.6 % and 30 % respectively, this is due to the time of concentration in the Soxleth equipment.
Table 1 Yields per 20 g of sample
| Sample | Extract | Extract volume (mL) | Yield % (w/w) |
|---|---|---|---|
| 1 | L. tridentata (2014) | 35 | 11.66 |
| 2 | L. tridentata diluted (2015) | 90 | 30 |
| 3 | O. vulgare (2014) | 41.5 | 13.83 |
| 4 | O. vulgare (2015) | 30 | 10 |
| 5 | A. ludoviciana (2014) | 36 | 12 |
| 6 | A. ludoviciana diluted (2015) | 105 | 35 |
| 7 | R. graveolens (2014) | 31.5 | 10.5 |
For extracts of O vulgaris, the yields were 13.8 and 10 %. Albado et al. (2001), report different yields according to the time and mode of the process, from its collection to its extraction. For A. ludiviciana the highest yield was in extract six, with 35 %, and the lowest in extract five with 12 %. Finally, the yield of R. graveolans extract was 10.5 %.
The prepared extracts were analyzed by gas chromatography to know their chemical composition. The running time of the standards in the CG was 18 min. with a retention time for terpinene of 3,524 min., limonene 3.62 min., linalool 4,218 min., thymol 8,870 min. and carvacrol 9.456 min. The injection volume for both standards and samples was 2 μL. Table 2 shows the concentrations used of the standards for the determination of the concentration in the samples, which allowed to perform a calibration curve of the signal measured as a function of the concentration of the analyte. The calibration allowed to estimate the parameters to determine the linearity of that curve, and consequently the capacity of an analytical method to obtain results, that were directly proportional to the concentration of the compound in the sample. Table 3 shows the chemical composition of the extracts of L. tridentata O. vulgare, A. ludoviciana and R. graveolens.
For the chemical composition, carvacrol, thymol, terpinene, linalool and limonene were determined. In the extracts one, two and three of L. tridentata, the thymol and carvacrol compounds were found in thymol concentrations of 4.30 mg ml-1 and 0.30 mg ml-1 respectively, obtained by using the equation of the linear regression of the standards used. While carvacrol concentrations were 7.79 mg ml-1 and 4.43 mg ml-1, the concentration of this compound being higher. Parts of medicinal plants (roots, leaves, branches, barks, flowers and fruits) are commonly rich in terpenes (carvacrol, citral, linalool and geraniol) and phenolic compounds (flavonoids and phenolic acids), and these have been effective as food additives (Cai et al., 2004).
Table 2 Concentrations of standards used for the chemical determination of extracts of Larrea tridentata, Origanum vulgare, Artemisa ludoviciana and Ruta graveolens by gas chromatography.
| Standard | Thymol (mg ml-1) | Carvacrol (mg ml-1) | Linalool (mg ml-1) | Terpinene (mg ml-1) | Limonene (mg ml-1) |
|---|---|---|---|---|---|
| 1 | 10.37 | 8.28 | 7.74 | 7.15 | 8.49 |
| 2 | 5.18 | 4.14 | 3.87 | 3.57 | 4.24 |
| 3 | 2.59 | 2.07 | 1.93 | 1.78 | 2.12 |
| 4 | 1.29 | 1.03 | 0.96 | 0.89 | 1.06 |
| 5 | 0.64 | 0.51 | 0.48 | 0.44 | 0.53 |
| 6 | 0.32 | 0.25 | 0.24 | 0.22 | 0.26 |
Table 3 Chemical composition by gas chromatography
| Sample | Extract | Terpinene (mg mL-1) | Limonene (mg mL-1) | Linalool (mg mL-1) | Thymol (mg mL-1) | Carvacrol (mg mL-1) |
|---|---|---|---|---|---|---|
| 1 | L. tridentata (2014) | 0 | 0 | 0 | 4.303 | 7.798 |
| 2 | L. tridentata diluted (2015) | 0 | 0 | 0 | 0.302 | 4.434 |
| 3 | O. vulgare (2014) | 0 | 0.066 | 0.125 | 4.185 | 9.102 |
| 4 | O. vulgare (2015) | 0.063 | 0 | 0.100 | 4.360 | 10.750 |
| 5 | A. ludoviciana (2014) | 0.051 | 0 | 0.288 | 0.025 | 0.049 |
| 6 | A. ludoviciana diluted (2015) | 0 | 0 | 0.116 | 0.013 | 0.023 |
| 7 | R. graveolens (2014) | 0 | 0.060 | 0 | 0 | 0 |
In extracts of O. vulgaris it was found that extract three, between its composition is limonene (0.6 mg ml-1), linalool (0.125 mg ml-1), thymol (4.18 mg ml-1) and carvacrol (9.10 mg ml-1); extract four was found terpinene at a concentration of 0.063 mg ml-1, but not limonene. Albado et al. (2001), reports on oregano oils the presence of terpineols, phenols and metabolically related compounds with 9 % carvacrol and 12.19% terpinene.
Regarding the chemical composition of the extracts of A. ludoviciana, extract five was found: terpinen (0.051 mg ml-1), linalool (0.288 mg ml-1), thymol (0.025 mg ml-1) and carvacrol (0.049 mg ml-1). For extract six the compounds were linalool (0.116 mg ml-1), thymol (0.013 mg ml-1) and carvacrol (0.023 mg ml-1). Kordali et al. (2005) investigated the chemical composition of A. ludoviciana, found that the extract contains mainly anethole (81%), beta-ocimene (6.5%), limonene (3.0%) and methyleugenol (1.8%); none of these reported compounds was identified in the present work. In the extract of R. graveolans limonene was found. From Feo et al. (2002) report that terpenoids constitute 11.2% of the oil of R. graveolans with α-pinene (1.3%), limonene (3.0%) and cineole (2.9%), as the main constituents of monoterpenes. The results coincide with the authors in the determination of limonene, but not in the concentration.
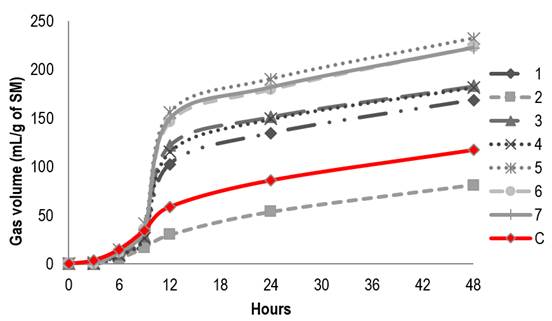
Figure 1 shows the kinetics of ruminal degradation applying for the modification of the fermentation of extracts of L. tridentata O. vulgare, A. ludoviciana and R. graveolens plants. In the in vitro gas production technique, the extract of L. tridentata was observed at 100 μL higher gas production in extract one (168.9 ml g-1) (Figure 1); but as the concentration of the extract increased, it decreased, being 665 μL of 50 mL g-1 (Figure 3).
In the digestibilities for extracts of O. vulgaris at 100 μL, the highest gas production was reported in extract three (98.54 ml g-1), but as the concentration of the extract increased, gas production also increased, to 665 μL of 180.06 ml g-1. For extract four the gas production was (665 μL), of 181.41 mL g-1. For the digestibilities of A. ludoviciana extracts at 100 μL, 330 μL (Figure 2) and 665 μL in gas production in extracts five and six was very similar 232 ± 27 mL g-1; in these digestibilities a change is not seen if more or less volume is added. Finally, extract seven of R. graveolans reports a volume of 222.39 ml g -1 at 100 μL, 330 μL 219.19 ml g-1 and 665 μL 210.86 ml g-1 being greater than the alfalfa control used (117.23 ml g-1).
Table 4 shows the means for the groups in the homogeneous subsets and the significant differences that exist between the different extracts and the applied doses of each one of them; confirming statistically that the gas (mg/ml) that was produced showed a statistically significant difference (p <0.05), since the highest gas production was obtained in the extract dose (330 μL) of A. ludoviciana.
Secondary compounds of plants, are recognized as antimicrobial agents that act against bacteria, protozoa and fungi. Phenolic compounds are the main active components (Dorman and Deans, 2000, Burt, 2004), and antibacterial activity can be given by a variety of non-phenolic substances (Newton and Van 2002; Burt, 2004).
Table 4 Analysis of means of gas production with the different doses of the seven extracts used.
| Extract | Extract dose (µL) | Produced gas (mL/g) ± *DE | |||||
|---|---|---|---|---|---|---|---|
| 3 hours | 5 hours | 9 hours | 12 hours | 24 hours | 48 hours | ||
| 1 | 100 | 0.10±0.12a | 8.49±0.74b | 23.08±1.59b | 102.88±7.35b | 134.75±10b | 168.91±12.2b |
| 2 | 100 | 0.00±0.00a | 5.91±0.13ª | 16.91±0.63ª | 30.14±0.71a | 53.85±0.68ª | 81.62±0.69a |
| 3 | 100 | 0.23±0.10ab | 9.78±0.30b | 28.44±0.84c | 121.86±2.29c | 151.36±2.39b | 183.07±2.28b |
| 4 | 100 | 0.00±0a | 9.18±0.28b | 26.8±1.07bc | 115.12±0.6bc | 148.97±0.43b | 181.42±2.89b |
| 5 | 100 | 1.18±0.26c | 14.67±0.93d | 41.22±1.99e | 155.90±10.4d | 190.53±11.7c | 232.41±17.6c |
| 6 | 100 | 0.45±0.45ab | 12.48±0.95c | 35.69±2.20d | 145.93±8.92d | 179.67±12.9c | 223.46±19.4c |
| 7 | 100 | 0.65±0.29bc | 12.25±1.18c | 35.08±2.50d | 149.30±6.98d | 181.77±10.3c | 222.40±20.4c |
| 1 | 330 | 0.28±0.43ª | 4.22±0.43ª | 10.02±0.69ª | 38.42±2.94ª | 49.56±3.23ª | 61.10±2.90ª |
| 2 | 330 | 0.28±0.43ª | 4.22±0.43ª | 10.02±0.69ª | 38.42±2.94ª | 134.50±6.33ª | 61.10±2.90ª |
| 3 | 330 | 0.19±0.19ª | 7.61±0.50b | 22.46±1.54b | 101.87±6.52b | 105.94±1.16c | 170.94±7.59c |
| 4 | 330 | 0.08±0.10ª | 6.69±0.36ab | 19.78±0.71b | 83.32±0.89b | 205.23±7.36b | 142.32±0.44b |
| 5 | 330 | 1.62±0.09b | 16.19±0.29d | 43.25±0.78d | 168.97±5.87d | 187.11±17.8e | 251.01±4.87d |
| 6 | 330 | 0.63±0.55ab | 12.71±0.99c | 35.71±1.86c | 152.67±19cd | 180.56±4de | 250.71±16.2e |
| 7 | 330 | 0.89±0.80ab | 13.98±2.8cd | 38.40±5.3cd | 143.68±3.05c | 130.35±61.2e | 219.19±5.16e |
| 1 | 665 | 0.00±0a | 1.47±0.19ª | 7.69±0.31ª | 34.55±1.48ª | 42.10±1.51ª | 50.88±1.64ª |
| 2 | 665 | 2.04±0.05cd | 9.32±0.05c | 22.9±0.45c | 40.94±0.36ab | 74.17±0.79b | 126.94±1.92c |
| 3 | 665 | 0.00±0a | 2.43±0.22ab | 8.03±0.20ª | 45.13±3.20b | 69.22±3.47b | 98.55±8.55b |
| 4 | 665 | 0.00±0a | 3.68±0.36b | 10.2±0.64b | 54.74±1.96c | 72.20±2.07b | 97.11±1.99b |
| 5 | 665 | 2.16±0.52d | 14.96±1.06e | 39.5±1.71e | 151.64±6.46e | 189.94±7.08c | 226.56±9.86e |
| 6 | 665 | 0.96±0.29ab | 11.83±0.67d | 32.8±1.25d | 131.39±1.25d | 164.40±2.55c | 226.69±4.47e |
| 7 | 665 | 1.08±0.9bc | 12.13±0.8d | 33.6±0.67d | 135.46±6.13d | 171.72±5.82c | 210.87±6.03d |
* DE: Standard deviation, values of means with different letters in the same column and in the same dose differ statistically (p<0.05).
The antimicrobial mode of action is mainly considered by the potential in the membrane of the bacterial cell to disintegrate the structures of this, which causes the leakage of ions; its effects on the activity of ruminal microorganisms depends on the species of plants consumed and their chemical composition. Monoterpenes, particularly monoterpene alcohols and aldehydes, strongly inhibit the growth and metabolism of rumen microbes; while monoterpene hydrocarbons slightly inhibit and sometimes stimulate the rumen's microbial activity (Benchaar et al., 2008). The effects of these compounds are modulated by the pH of the rumen, the diet in which they are included, and by preparation and extraction methods (Patra and Saxena, 2010; Patra and Yu, 2014).
It has been reported that plant extracts and oils inhibit bacteria producing ammonia nitrogen, thus decreasing the deamination of amino acids, mainly in diets containing not very high amounts of protein (Patra and Saxena, 2010). It has also been observed that the total number of viable bacteria is not affected, ie only the proportions of bacterial groups change; but in in vitro studies where high doses have been included, such as 400 μg ml-1 of thymol compared with those commonly reported in the literature, they cause a decrease in the total number of microorganisms (McKay and Blumberg, 2006). The modifications in the total and proportion of the VFA impact on the production profiles of methane, especially if it reduces the proportion of acetate and increases the propionate; therefore, changes in these will have a positive or negative impact on methane production.
In volume of 100 μL per 100 ml of ruminal fluid, at the end of the digestibility sample was taken for the determination of VFA; the results are shown in table 5. Also, the methane production obtained by the stoichiometric equation is reported; from Moss et al. (2000), finding the highest production in methane with the extract three of O. vulgaris with 1162 g L-1, and the minimum in the extract of A. ludoviciana 0.204 g L-1.
For the sample where 300 μL per 100 ml of ruminal fluid was added, the results are as follows, the highest digestibility is reported in samples five and six corresponding to the extract of A. ludoviciana. Table 5 shows the results of AGV and methane production, with the extracts six of A. ludoviciana (1030 g L-1) and seven of R. graveolans (1029 g L-1) the highest production generated in this digestibility. For the 665 μL dose, the results obtained are as follows; greater digestibility is reported in samples five and six corresponding to the extract of A. ludoviciana. Table 5 shows the concentrations of the VFA and the methane production, being higher in the extract five of A. ludoviciana (1148 g L-1) and the lower production of methane is reported in the extract one of L. tridentata generating 0.193 g L -1.
Table 5 Production of VFA and methane at 48 h of ruminal digestibility applying 100, 330 and 665 μL of extract
| Acetic acid | |||
|---|---|---|---|
| g L -1 ± DE | |||
| 100 µL | 330 µL | 665 µL | |
| Control | 1.67±0.00006d | 1.67±0.00006d | 1.67±0.00006bc |
| 1 | 1.72±0.00006e | 0.90±0.00012a | 0.6±0.00013a |
| 2 | 2.41±0.00002h | 1.73±0.00016e | 1.83±0.00021c |
| 3 | 2.14±0.00024g | 1.28±0.00017b | 0.82±0.71442a |
| 4 | 1.80±0.00007f | 1.31±0.00009c | 1.01±0.00009ab |
| 5 | 0±0a | 1.74±0.00006f | 1.99±0.00027c |
| 6 | 0.62±0.00013b | 1.84±0.00026g | 1.97±0.00009c |
| 7 | 1.59±0.00007c | 1.86±0.00005h | 1.94±0.00005c |
| Propionic acid | |||
| Control | 0.923±0.00017c | 0.923±0.00017b | 0.923±0.00017d |
| 1 | 0±0a | 0±0a | 0.436±0.00020b |
| 2 | 1.19±0.00016d | 0±0a | 0±0a |
| 3 | 0±0a | 0±0a | 0±0a |
| 4 | 0±0a | 0.94±0.00017c | 0±0a |
| 5 | 0±0a | 0±0a | 0±0a |
| 6 | 0±0a | 0±0a | 0±0a |
| 7 | 0.74±0.00018b | 0±0a | 0.883±0.00018c |
| Butyric acid | |||
| Control | 0.036±0.00022b | 0.037±0.00022b | 0.037±0.00022a |
| 1 | 0.434±0.00013e | 0.37±0.00015d | 0.016±0.00022a |
| 2 | 0.053±0.00021c | 0.424±0.00014e | 0.437±0.00013bc |
| 3 | 0.492±0.00012f | 0.349±0.00015c | 0.413±0.15101bc |
| 4 | 0.539±0.00011h | 0.014±0.00022a | 0.336±0.00016b |
| 5 | 0.509±0.00012g | 0.444±0.00013f | 0.622±0.00010d |
| 6 | 0.076±0.00021d | 0.482±0.00012h | 0.508±0.00012cd |
| 7 | 0.029±0.00022a | 0.469±0.00013g | 0.037±0.00022a |
| Methane | |||
| Control | 0.514±0.00007c | 0.515±0.00007b | 0.515±0.00007b |
| 1 | 0.951±0.00008f | 0.554±0.00011c | 0.193±0.00009a |
| 2 | 0.777±0.00005e | 0.95±0.00012e | 1.002±0.00014c |
| 3 | 1.162±0.00010h | 0.716±0.00010d | 0.536±0.26108b |
| 4 | 1.03±0.00007g | 0.338±0.00008a | 0.59±0.00010b |
| 5 | 0.204±0.00005a | 0.964±0.00008f | 1.148±0.00011c |
| 6 | 0.312±0.00014b | 1.021±0.00016g | 1.09±0.00006c |
| 7 | 0.525±0.00007d | 1.029±0.00007h | 0.648±0.00006b |
* DE: Standard deviation, values of means with different letters in the same column and in the same dose differ statistically (p <0.05).
Regarding the production of AGV, the lowest production of acetic acid was reported in extract one with 665 μL, being 0.680 g L-1 (gas production 50 ml g-1); while that extract two had the highest concentration at 100 μL (2.411 g L-1); being this one that smaller amount of gas produced to this concentration (81.62 ml g-1). For propionic acid the production of this VFA is inhibited, only found in the extract two to 100 μL (1197 g L-1), and in the extract one to 665 μL (0.436 g L-1). Butyric acid showed a concentration not higher than 0.622 g L-1 100 μL, 330 μL and 665 μL. As for the calculated methane production, one was lower at 330 μL (0.554 g L-1) and at 665 μL (0.193 g L-1) compared to the standard alfalfa that produced 0.514 g L-1 methane; the highest methane production is reported at 665 μL in extract two (1,002 g L-1), this being the highest gas production (126.94 ml g-1) to this volume of extract. Agarwal et al. (2009) and Cobellis et al. (2016), report that by adding extracts and oils from plants in the production of AGV, these are modified; they report that the acetate-propionate ratio increases; although in other studies no differences have been found when adding extracts (Ravindra et al., 2009, Wang et al., 2009).
The lowest production of acetic acid was reported in extract three with 665 μL, being 0.825 g L-1 (gas production 180.54 ml g-1); while extract three had the highest concentration at 100 μL (2.145 g L-1), this being one of the least gas produced (98.54 ml g-1).
For propionic acid the production of this AGV is inhibited, only found in the extract four to 330 μL (0.947 g L-1) and a gas production of 143.32 mL g-1. While butyric acid showed a concentration greater than 0.539 g L-1 at 100 μL, and the lowest concentration was reported in extract four at 330 μL (0.014 g L-1). As for the methane production calculated, it was lower in the extract three to 665 μL (0.536 g L-1), and in the extract four to 665 μL (0.590 g L-1), with respect to the control of alfalfa that produced 0.514 g L-1 methane. The highest methane production is reported at 100 μL in extract three (1162 g L-1), this being one of the lowest gas production (98.54 mL g-1) in this extract volume.
Extract six with 100 μL showed an acetic acid concentration of 0.625 g L-1 (gas production 223.45 ml g-1), while extract five showed the highest concentration at 665 μL (1997 g L -1), with a gas production of 226.54 mL g-1. The production of this VFA is inhibited for propionic acid. While butyric acid presented concentrations not higher than 0.746 g L-1 at 100 μL, 330 μL and 665 μ. The lowest production is observed at 100 μL in extract six (0.076 g L-1).
As for the calculated methane production, it was lower in the extract five to 100 μL (0.204 g L-1) and in the six to 100 μL (0.312 g L-1), with respect to the alfalfa control that produced 0.514 g L-1 methane. The highest production of methane is reported at 665 μL in extract five (1148 g L-1), this being the highest gas production (226.54 mL g-1) at this volume.
In the extract of R. graveolans at 100 μL, the production of acetic acid was reported from 1596 ml g-1 (gas production 222.39 ml g-1), to 330 μL 1,869 g L-1 (gas production 219.19 ml g-1) and 665 μL of 1,947 mL g-1 (gas production 210.86 mL g-1); while the standard of alfalfa reports 0.923 ml g-1, being higher that found in the extract seven to 330 μL and to 665 μL. For propionic acid production is at 100 μL of 0.746 g L-1 and 665 μL of 0.883 g L-1; while butyric acid showed a concentration greater than 0.469 g L-1 at 330 μL; the lowest production is observed at 100 μL of 0.029 g L-1. As for the calculated methane production, it was less than 100 μL (0.525 g L-1) (where the greatest amount of gas was produced 222.39 ml g-1) and 330 μL produced 1,029 g L-1; the standard of alfalfa produced 0.515 g L-1 of methane, which indicates that there was no decrease of this with the extract of R. graveolans.
Because of 40 to 60 % of the total greenhouse gases (GHG) of livestock come from enteric fermentation, manure management and different activities related to obtaining food for animals (Sejian et al. , 2015); A strategy to be followed and considered for mitigation of methane should be focused on the modification of ruminal fermentation without altering animal production, improving feed conversion.
CONCLUSION
O. vulgaris extract with a higher concentration of thymol and carvacrol showed better gas digestibility, lower AGV production and lower methane concentration using volumes 330 and 665 μL; therefore, we consider it necessary to continue investigating the use of the extracts studied as additives in in vivo tests.











 texto em
texto em