Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Abanico veterinario
versión On-line ISSN 2448-6132versión impresa ISSN 2007-428X
Abanico vet vol.9 Tepic 2019 Epub 05-Mar-2021
https://doi.org/10.21929/abavet2019.94
Original Article
Leukocytes during embryonic development on ewes as a response to organic selenium
1Postgraduate College-Campus Montecillo. Edo from Mexico. Mexico. ma.monserrat_love@hotmail.com, lmirandaj@colpos.mx, queroadrian@colpos.mx, arobledo@colpos.mx
2Productive companies, AGRIBEST Technologies, S.A. of C.V. Edo from Mexico. Mexico. smorales@colpos.mx
Selenium decreases cellular damage caused by oxidative stress and improves the organism physiological response. Leukocytes were evaluated during embryonic development in ewes with consumption of organic selenium. A total of 27 local ewes at the municipality of San Andrés Chiautla, Mexico State, with an average age of 2.5 years, were randomly assigned in two groups, 11 in control group (without Selenium, S-Se) and 16 with oral selenium (C-Se; selenium-enriched yeast) addition at dose of 0.3 ppm, for a period of seven days before Artificial Insemination (AI) consuming a diet based on alfalfa and corn stubble. White cell differential counting was performed on blood smears. Samples were taken before, during and after selenium treatment. Data were analyzed by mixed model, showing significant differences by treatment and time (p ≤ 0.05) in neutrophils; day 9 of embryonic development (5.91 ± 0.89 y 2.11 ± 0.46; S-Se y C-Se, respectively) and basophils amount was at day -3 (1.85 ± 0.31 y 1.05 ± 0.23). Regarding lymphocytes and eosinophils, they showed a difference in time (p ≤ 0.05) but not by treatment (p ≥ 0.05). It is concluded that selenium decreases the number of neutrophils and basophils; after blastocyst formation and four days after starting organic selenium treatment.
Keywords: leukocytes; organic selenium; ewes
El selenio contrarresta el daño celular causado por estrés oxidativo y mejora la respuesta fisiológica del organismo. En este estudio se evaluaron leucocitos durante el desarrollo embrionario de ovejas como respuesta al selenio orgánico. Veintisiete ovejas locales del municipio de San Andrés Chiautla, Estado de México, con edad promedio de 2.5 años, se asignaron al azar en dos grupos, 11 en grupo testigo (sin selenio; S-Se) y 16 con adición de levadura enriquecida con selenio (selenio orgánico; C-Se) a dosis de 0.3 ppm, vía oral por período de siete días antes de la Inseminación Artificial (IA) consumiendo una dieta a base de alfalfa achicalada y rastrojo de maíz. Se realizó conteo diferencial de células blancas; en frotis sanguíneos realizados antes, durante y después del tratamiento de selenio. Los datos se analizaron mediante modelo mixto, mostrando diferencias significativas por tratamiento y tiempo (p ≤ 0.05) en neutrófilos; el día 9 del desarrollo embrionario (5.91 ± 0.89 y 2.11 ± 0.46; S-Se y C-Se, respectivamente) y basófilos el día -3 (1.85 ± 0.31 y 1.05 ± 0.23). En cuanto a los linfocitos y eosinofilos mostraron diferencia por tiempo (p ≤ 0.05) pero no por tratamiento (p ≥ 0.05). Se concluye que el selenio disminuye el número de neutrófilos y basófilos; después de formarse el blastocisto y cuatro días después de iniciado el tratamiento con Selenio.
Palabras clave: leucocitos; selenio orgánico; ovejas
INTRODUCTION
Selenium (Se) is an essential micronutrient necessary for the proper functioning of the organism, deficiency in this mineral produces pathologies (Huang et al., 2012; Ahsan et al., 2014). Selenium levels regulate inflammation and immunity processes (Huang et al., 2012). There are two types of response to the invasion of foreign agents to the organism: The innate (natural) immune response; it is a rapid, non-specific response, composed of mechanical barriers, mucous membranes and cells that produce cytokines and chemokines. Innate immunity acts organically until the activation of adaptive immunity. Acquired (adaptive) immunity is a rapid response, with immunological memory, mediated by T and B cells, which for their activation require the presentation and processing of antigens. Adaptive immunity is divided into two areas, humoral immunity mediated by B lymphocytes and cellular immunity mediated by T lymphocytes. Innate and acquired responses generally work together to eliminate pathogens. The innate response cells use phagocytic cells (neutrophils, monocytes and macrophages), cells that release inflammatory mediators (basophils, mast cells and eosinophils) and natural killer cells (Delves and Roitt, 2000).
According to Martínez et al., (2011) during normal gestation in the uterine mucosa a large number of leukocytes (neutrophils, macrophages, natural killer cells and dendritic cells) are found; cells that fulfill multiple functions such as: phagocytosis, production of cytokines, production of oxygen metabolites (nitric oxide (NO), superoxide anion), release of prostaglandins, acute phase proteins and antimicrobial peptides.
Research done in recent years involves aspects of the immune system (in selection, maturation and activation of some events of immune cells), these studies show variations in leukocyte concentrations according to the physiological events of focus, such as: gestation, lactation, age, race, stress effect and even the geographical location of the animal exploitation; as well as the state of health (Hoffmann and Berry, 2008).
The amount of nutrients transferred to the offspring depends on the nutritional status of the mother and the efficiency of the transplacental and mammary transport mechanism; the passage of trace elements and other nutrients through the placenta is necessary for: embryonic development, fetal growth and physiological functions of the mother and fetus during pregnancy. Selenium passes efficiently through the placental barrier into fetal tissues and is also transferred to colostrum and milk (Rock et al., 2001, Stewart et al., 2012, Erdogan et al., 2017). The nutritional deficiency of selenium causes: fertility problems, abortion, placental retention, white muscle disease, neonatal weakness (Spears, 2011), mortality, growth, development and implantation of the inadequate embryo (Sharma and Agarwal, 2004) and low immunity (Rayman, 2012). These problems can be prevented by adding selenium to the animals' diet.
There are different forms and sources of selenium; organic (selenomethionine, selenocysteine) and inorganic (selenite and sodium selenate). The organic has greater bioavailability, is three times less toxic than inorganic; it is cytoprotective, because it is incorporated into the reserve tissues (Yue et al., 2009; Lyons et al., 2007). However, despite the fact that several authors mention the benefits of selenium in cellular immunity, there is no precise information on the effect of the mineral in the presence of cells of the white cell pack; responsible for immune response in ewe in gestation process.
Therefore, the objective of the study was to evaluate the response of leukocytes during the embryonic development of sheep with oral application of organic selenium given before being inseminated.
MATERIAL AND METHODS
The investigation was carried out in the San Andrés Chiautla municipality, State of Mexico; with geographic coordinates 19 ° 36'19 "N and 98 ° 54'38" W, and altitude of 2260 m. The climate is temperate-semi-dry, registering an annual average temperature of 19 ° C, with a maximum of 32 °C and a minimum of 6 °C (Inafed, 2018). We used 27 local sheep (indefinite breed) multiparous with weight between 35 and 38.2 kg, body condition of 2.5 (scale of 1 to 5), with an average age of 2.5 years. The sheep were randomly assigned in two groups, 11 for the control group (without selenium, S-Se) and 16 with addition of yeast enriched with selenium (organic selenium, C-Se) in a dose of 0.3 ppm; requirement according to the NRC, considering that the place where the experiment was conducted is deficient in selenium (Targetmap, 2013). During the whole experiment the sheep were kept in permanent stabling and fed a diet based on reduced alfalfa and corn stubble.
The oral administration of organic selenium (C-Se) was per period of seven days before the Artificial Insemination (AI), and the group S-Se were administered placebos. Before the start of treatment, they were dewormed (Ivermetin dose: 1ml) and vitamin was applied (Complex B); they underwent oestrus synchronization protocol with intravaginal sponges Cronolone (Chronogest® CR), impregnated with 20 mg of progestogen for 14 days. When removing the sponges, 400U I of eCG (Folligon®) was applied and Artificial Insemination (AI) was performed 55 hours after the removal of sponges, by the endoscopy method.
Blood samples were taken to perform smears; before treatment with organic selenium, before deworming the sheep (39 days before AI) and one day before starting selenium treatment, during days -5, -3 and -1; considering day zero as the day of Artificial Insemination (AI) and after; on days 1, 3, 5, 7, 9, 11, 13, 19, 23, 28 and 42; this last sampling period was considered as corresponding to the embryonic development of the sheep.
The technique used for this study is the one used by the reproduction module of the Postgraduate School. The blood smears were made by placing a drop of blood in the center of the slide and the sample was scanned uniformly to form a thin layer of blood tissue; dried at room temperature for approximately 10 minutes; Subsequently, 96% ethanol was applied to fix the sample, stained with Hematoxylin Gill No.3 (Sigma-Aldrich) for 5 minutes; After that, a wash with distilled water was carried out.
The differential leukocyte count was performed with an optical microscope at 40x resolution with five repetitions (microscopic fields) per sample. The diagnosis of pregnancy was made 35 days after insemination with Draminski portable ultrasound scanner model 4Vet mini, with 7.5 MHz abdominal transducer; the exploration of structures of gestation was made at the level of the iliac fossa. For the diagnosis, the sheep were fed 12 hours before performing the ultrasound.
The dependent variables evaluated were differential amount of white blood cells (lymphocytes, neutrophils, monocytes, basophils and eosinophils). The sheep were randomly distributed for each treatment; including the sheep as a random effect. A mixed model was used by the MIXED procedure (SAS, 2002), with the following model: Y ijk = μ + T i +Time j +T*Time ijk +ε ijk ; where: Y ij : dependent variable analyzed, μ: general average of the population, T i : i-th treatment, P j : j-th time, T* Tiempo ij : interaction between treatment and sampling time, ε ijk : random error. In the cases of statistical significance (p ≤ 0.05), the means were compared with the Tukey test.
RESULTS AND DISCUSSION
In the present study, the addition of organic selenium to sheep did not show significant differences (p ≥ 0.05) in lymphocytes (figure 1a); whereas there was a significant effect by treatment (p ≤ 0.05) in neutrophils on day 9 of embryonic development (5.91 ± 0.89 S-Se and 2.11 ± 0.46 C-Se, respectively (figure 1b)). No significant difference (p ≥ 0.05) was found in lymphocytes between treatments; in contrast to the results found by Broome et al., (2004), who when adding selenium observed an increase in the number of lymphocytes. The day -8 corresponding one day before the start of treatment and day -5 during treatment; they show the smallest number of white cells in time. Day one corresponds one day after insemination, and on day three the ovule is fertilized; thus observing a decrease in lymphocytes and neutrophils in both physiological events. Whereas neutrophils are phagocytic cells (along with monocytes and macrophages), they would be linked to the possibility of reduced sperm rejection.
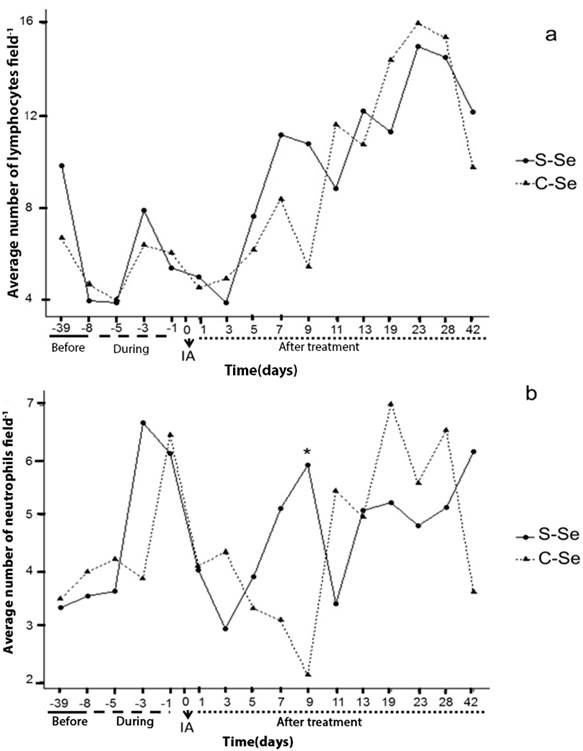
S-Se: without selenium, C-Se: yeast enriched with organic Selenium, dose (0.03 ppm); -39, -8: days before the start of treatment; -5, -3, -1: samples during the treatment; 1: beginning of pregnancy; 1, 3, 5, 7, 9, 11, 13, 19, 23, 28 and 42: days of the embryonic development of the sheep. IA: Artificial insemination. *: Indicates significant differences (P <0.05).
Figure 1a and 1b Behavior of lymphocytes and neutrophils over time in local sheep (indefinite race).
T lymphocytes are considered modulators of the mother's immune response, since they regulate the implantation process during the first trimester of pregnancy, with an increase in T cells (Martínez et al., 2011). Results that coincide in this study by increasing the number of lymphocytes from day 11 of embryonic development, where days after implantation begins (figure 1a). In spontaneous abortions, a decrease in the number of regulatory T cells has been observed (Guerin et al., 2009).
The white cells that showed the highest percentage in this investigation were lymphocytes (55.06%), neutrophils (29.22%) and monocytes (8.26%); and in smaller quantity the basófilos and eosinófilos with 0.70% and 4.46%, respectively. Regarding the treatments, a slight elevation of average values was shown in the addition of organic selenium (C-Se) with 58.0%, and without selenium (S-Se), 41.95%, although there were no statistical differences (p ≥ 0.05); this may be due to external and internal events of the animal, such as normal or pathological physiology. These results agree with Yue et al., (2009) and Brown et al., (2000), that when evaluating different treatment groups with selenium, there were no significant differences in the variables evaluated. Brown et al. (2000), mentions that despite the numerical variation of the values, the no difference is due to variation in interindividual responses.
Regarding the basophils, significant difference was observed (p≤0.05) between treatment and in time; day -3 showed decrease in the average value of basophil cells for the treatment with organic selenium (1.05 ± 0.23), in comparison with the control group S-Se (1.85 ± 0.31). On day 5, 7 and 9 of the embryonic development, a decrease in basophils and eosinophils was observed (figure 2a, b). These days correspond to the moment of blastocyst formation (5-6 days) and an inflammatory stage begins, according to what Delves and Roitt (2000) describe, these cells (basophils and eosinophils) are inflammatory mediators. Canalejo et al., (2007) found no differences in basophils, eosinophils and lymphocytes during the gestation period, despite this being a stage with the presence of uterine tissue inflammation. In diseases such as allergic inflammation, numbers of eosinophils increase markedly in the blood and tissues, where inflammation is localized (Davoine and Lacy, 2014).
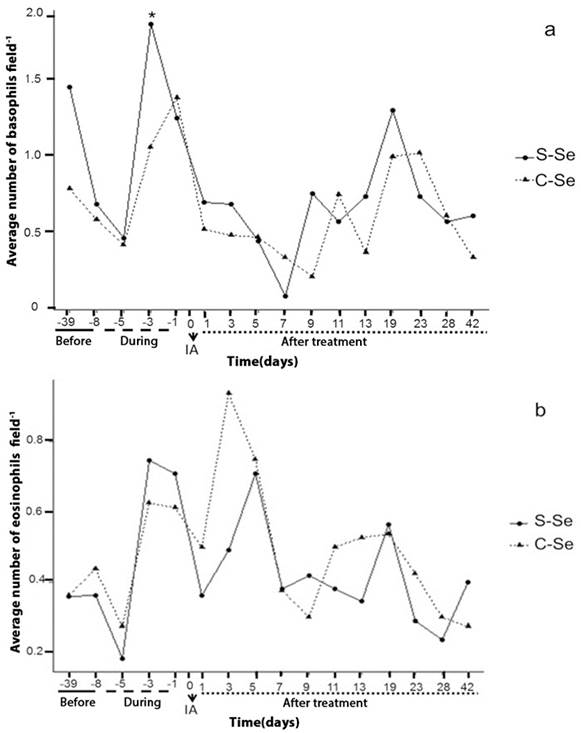
S-Se: without selenium, C-Se: yeast enriched with organic Selenium, dose (0.03 ppm); -39, -8: days before the start of treatment; -5, -3, -1: samples during the treatment; 1: beginning of pregnancy; 1, 3, 5, 7, 9, 11, 13, 19, 23, 28 and 42: days of the embryonic development of the sheep. AI: Artificial Insemination *: Indicates significant differences (P <0.05).
Figure 2a and 2b Behavior of basophils and eosinophils over time in local sheep (indefinite race).
From day 11 of the embryonic development the values of the white cells increase (figure 1 and 2), considering that the following days physiological processes occur; on day 13 embryonic implantation occurs in the sheep and the establishment of placentas is recorded for 30-80 days (Byers and Kramer, 2010). The increase recorded in white cells after the physiological events discussed here may not have a direct cause on the embryo, given that at this moment the placental barrier is developing and serves as a direct protection to the embryo.
CONCLUSIONS
It is concluded that the administration of organic selenium (0.3 ppm), decreases the number of neutrophils and basophils, after forming the blastocyst and four days after starting the treatment. Statistical differences by time are due to physiological factors of embryonic development. No statistical differences were found in lymphocytes, monocytes, eosinophils.
LITERATURA CITADA
AHSAN U, Kamran Z, Raza I, Ahmad S, Babar W, Riaz MH, Iqbal Z. 2014. Role of selenium in male reproduction: a review. Anim. Reprod. Sci. 146(1-2):55-62. DOI: 10.1016/j.anireprosci.2014.01.009. [ Links ]
BROOME CS, McArdle F, Kyle JA, Andrews F, Lowe NM, Hart CA, Arthur JR, Jackson MJ. 2004. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am. J Clin Nutr. 80(1): 154-162. DOI: 10.1093/ajcn/80.1.154. [ Links ]
BROWN KM, Pickard K, Nicol F, Beckett GJ, Duthie Gg, Arthur JR. 2000. Effects of organic and inorganic selenium supplementation on selenoenzyme activity in blood lymphoctyes, granulocytes, platelets and erythrocytes. Clinical Science. 98(5): 593-599. DOI: 10.1042/CS19990276. [ Links ]
BYERS SR, Kramer JW. 2010. Normal hematology of sheep and goats. In: Weiss DJ, Wardrop KJ, eds. Schalm's Veterinary Hematology. 6th ed. Ames, IA: Blackwell Publishing; 2010. p. 836-842. http://www.worldcat.org/title/schalms-veterinary-hematology/oclc/338288636. [ Links ]
CANALEJO K, Tentoni J, Aixalá M, Jelen AM. 2007. Valores de referencia del hemograma en embarazadas, con tecnología actual. Bioquímica y Patología Clínica. 71(2): 52-54. ISSN: 1515-6761. [ Links ]
DAVOINE F, Lacy P. 2014. Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity.Front. Immunol. 5:570. DOI: 10.3389/fimmu.2014.00570. [ Links ]
DELVES PJ, Roitt IM. 2000. The Immune System. The New England J. Med. 343(1):37-49. DOI:10.1056/NEJM200007063430107. [ Links ]
ERDOGAN S, Karadas F, Yilmaz A, Karaca S. 2017. The effect of organic selenium in feeding of ewes in late pregnancy on selenium transfer to progeny. Revista Brasileira de Zootecnia. 46(2):147-155. ISSN: 1806-9290. DOI: 10.1590/S1806-92902017000200010. [ Links ]
GUERIN LR, Prins JR, Robertson S. 2009. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment?. Hum Reprod Update. 15(5):517-535. DOI: 10.1093/humupd/dmp004. [ Links ]
HOFFMANN RP, Berry JM. 2008. The influence of selenium on immune responses. Mol. Nutr. Food Res. 52(11):1273-1280. DOI: 10.1002/mnfr.200700330. [ Links ]
HUANG Z, Rose AH, Hoffmann PR. 2012. The Role of Selenium in Inflammation and Immunity: From Molecular Mechanisms to Therapeutic Opportunities. Antioxidants & Redox Signaling. 16(7):705-743. DOI: 10.1089/ars.2011.4145. [ Links ]
INAFED (Instituto Nacional para el Federalismo y el Desarrollo Municipal) 2018. Enciclopedia de Los Municipios y Delegaciones de México. Disponible: http://www.inafed.gob.mx/work/enciclopedia/EMM15mexico/municipios/15028a.html. [ Links ]
LYONS MP, Papazyan TT, Surai PF. 2007. Selenium in food chain and animal nutrition: Lessons from Nature. Asian-Australian Journal of Animal Science. 20(7):1135-1155. DOI: 10.5713/ajas.2007.1135. [ Links ]
MARTÍNEZ AOA, Villaseñor EN, Kuribreña AJC, Vega SE. 2011. Modulación de la respuesta inmunológica durante el embarazo. Rev Cubana Obstet Ginecol. 37(2):277-287. ISSN 0138-600X. [ Links ]
RAYMAN MP. 2012. Selenium and human health. Lancet. 379(9822):1256-1268. DOI: 10.1016/S0140-6736(11)61452-9. [ Links ]
ROCK MJ, Kincaid RL, Carstens GF. 2001. Effects of prenatal source and level of dietary selenium on passive immunity and thermos metabolism in new born lambs. Small Ruminant Research. 40(2):129-138. DOI:https://doi.org/10.1016/S0921-4488(01)00167-5. [ Links ]
SAS, 2002. Versión 9.0 for Windows edition. SAS Institute Inc. Cary, NC, USA. [ Links ]
SHARMA RK, Agarwal A. 2004. Role of reactive oxygen species in gynecologic diseases. Reprod Med Bio. 3:177-199. DOI: 10.1111/j.1447-0578.2004.00068.x. [ Links ]
SPEARS JW. 2011. Selenium deficiency and its prevention in grazing ruminants. Salt and Trace Minerals. Disponible: http://saltinstitute.org/wp-content/uploads/2013/05/4th-qtr-2011.pdf [ Links ]
STEWART WC, Bobe G, Pirelli GJ, Mosher WD, Hall JA. 2012. Organic and inorganic selenium: III. Ewe and progeny performance. Journal of Animal Science. 90(12):4536-4543. DOI: 10.2527/jas2011-5019. [ Links ]
TARGETMAP. 2013. Distribución de selenio en México por Estado. Disponible: https://www.targetmap.com/viewer.aspx?reportId=25908 [ Links ]
YUE W, Zhang C, Shi L, Ren Y, Jiang Y, Kleemann O. 2009. Effect of supplemental selenomethionine on growth performance and serum antioxidant status in Taihang black goats. Asian-Aust. J. Anim. Sci. 22(3):365-370. ISSN: 1011-2367, DOI: 10.5713/ajas.2009.80474. [ Links ]
Received: February 23, 2018; Accepted: July 20, 2018











 texto en
texto en 



