INTRODUCTION
Sheep production in Mexico is carried out under traditional grazing systems, with low technology and low productivity. In it they are characterized and distinguished by regions; the north, which bases its production on sheep of wool and on breeds for meat with technified systems; the central region, which produces with crossed cattle (Suffolk or Hampshire and hair breeds) and it is carried out in an important way in marginalized areas, in pastures and in agricultural lands with agricultural residues. The southern and southeastern regions are described as having a tropical climate where hair breeds (Pelibuey and Black Belly) stand out, although specialized breeds for meat production have been incorporated (Dorper and Katahdin, Hernández-Marín et al., 2017 ).
Studies in sheep considered management practices to improve the productive efficiency of herds in a technical and economic way, in which it is intended to eliminate the pharmacological manipulation of animals (Martin et al., 2004). These methodologies are based on knowledge of reproductive events, socio-sexual factors and the effects of nutrition (Hawken and Martin, 2012, Scaramuzzi et al., 2013); because at present, reproductive management protocols are based on the application of exogenous hormones that simulate the action of a corpus luteum (CL), such as progestogens (P4); and others manage to eliminate it, to induce a follicular phase and ovulation, such as prostaglandins (PGF2α, Abecia et al., 2012). However, natural methods are also capable of inducing ovulation, such as the action of male pheromones ('male effect', Hawken and Martin, 2012).
The use of P4-releasing intravaginal devices (CIDR®) has been discussed, due to the alteration in the release of LH, the quality of ovulation, animal welfare and public health; therefore, it is necessary to generate protocols of short duration, with fewer doses and more effective release devices (Abecia et al., 2011). In this regard, an alternative reproductive management is to synchronize with PGF2α, because these are metabolized faster in the liver and do not accumulate in tissues (Davis et al., 1980). A protocol commonly used in sheep is to apply 125 mg of cloprostenol or 7.5 mg of luprostiol (Abecia et al., 2011). For their effectiveness they must be applied in the presence of a corpus luteum; while, the sheep in anoestrus, will not respond to the treatment. The administration of two doses of PGF2α is recommended to synchronize estrus in cycling sheep, with an interval of 9 to 10 d, which ensures that most females will present the middle luteal phase, when applying the second dose, and that all they will respond with the behavior of estrus and ovulation (Godfrey et al., 1999). However, their response may vary due to the insemination technique, the dose to be applied and the intervals between doses (Fierro et al., 2013).
Biostimulation is used to replace the function of exogenous hormones and improve reproductive efficiency in sheep (Hawken and Martin, 2012). Ungerfeld (2011) found that the "male effect" together with the second injection of PGF2α increased the behavior of estrus and the number of sheep in estrus, compared to two injections of PGF2α without "male effect". Thus, the introduction of sheep in previously isolated sheep induces an increase in the pulsatility of LH, which stimulates the secretion of estradiol to increase and triggering luteolysis (Meilán and Ungerferld, 2014).
Therefore, the objective of the present study was to evaluate the biostimulation with 'male effect' on the reproductive response of wool sheep with a protocol based on the application of prostaglandins.
MATERIAL AND METHODS
Location
The present study was carried out during the breeding season during the month of July 2015 in the Sheep and Goats Module of the Experimental Farm of the Zootechnics Department of the Chapingo Autonomous University, located in Texcoco, State of Mexico (19° 29'N, 98° 53'W and altitude of 2250 m). The climate is temperate subhumid with rain in summer, which is described as C (Wo)(W)b (i') g (García, 2004).
Management of experimental animals
24 Suffolk sheep with 5.1(1.2 years of age, 47.35(2.5 kg of weight and body condition of 3 units (scale 1 to 5 units, Russell et al., 1969) and 29 Rideau Arcott sheep with 6.3(1.8 years of age, 52.19(1.8 kg of weight and body condition of 3 units were used. During the development of the experiment, all the sheep received twice a day (7:00 AM and 4:00 PM) 2.5 kg sheep-1 d-1 of an integral diet made with ground hay of Avena sativa (70%), commercial concentrate which contained 15% crude protein and 2.9 Mcal of metabolizable energy kg-1 (30%), mineral salts and freely available water. Before allocating them to the treatments, all sheep were treated with 10.95 mg sodium selenite sheep -1 (Muse, Intervet®, Mexico) and dewormed with a combination of Ivermectin 0.2 mg kg-1 live weight (LW) and Closantel 5 mg kg-1 of LW (Oviver, Lapisa®, Mexico). All the animals were kept in pens provided with shade, feeding trough, automatic drinker and dirt floor, in quantity according to treatment.
Biostimulation with "male effect"
Prior to the stimulation of the ram, all the sheep were kept for 48 d at a minimum distance of 500 m from the pen of the males, to avoid visual, auditory and olfactory contact between them; and in this way, increase the stimulus at the time of contact. The "macho effect" consisted of introducing a sexually experienced adult Creole ram (4.6 years of age and 78.25 kg of average weight) provided with an apron (to avoid copulation) for 12 hours, in the corral of the T2 females from the fourth day, after the first application of PGF2(. The management of the experimental sheep and the stallions used in the biostimulation and in the application of the PGF2( was carried out in accordance with the Mexican norms NOM-024-ZOO-1995 and NOM-033-ZOO-1995 (SAGARPA, 2015).
Treatments and synchronization protocol
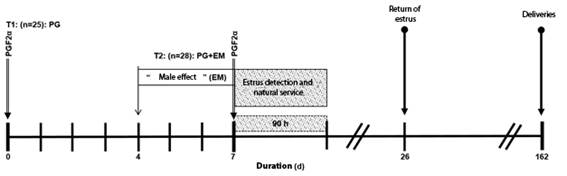
The sheep were randomly assigned to one of two treatments of the estrus synchronization protocol (T): T1, n = 25: synchronized sheep with two doses of 250 μg of Cloprostenol sheep-1 via IM (Celosil®, Intervet, Mexico) with interval of seven days (PG, Control); and T2, n=28: similar to T1, but with the "male effect" from fourth day to seventh day of the application of Cloprostenol (PG + EM, Figure 1).
Estrus detection and natural service
Four Suffolk rams with 4.6(1.3 years of age and 87.75(2.5 kg of weight were used, and two Rideau Arcott rams with 4.1(1.5 years of age and 91.38(3.7 kg of weight, all with proven fertility (Malejane et al., 2014), which were introduced with the females during 90 h, at intervals of 6 h, to detect estrus, after applying the second dose of PGF2α. It was considered that a sheep presented estrus when it was receptive to the male, performed lordosis behavior, was totally immobilized and accepted the natural montage. Once detected in estrus and served, each sheep separated from its group (to avoid preference for the male) and it was given a second mount at 12 hours later, to encourage the sheep to continue with the detection of the other sheep.
Return of estrus
After detecting the estrus and offering service to the sheep by natural one, the rams stayed away from the herd for 15 days. On day 16, a harness impregnated with a dye was placed on the chest and introduced again with the females, to evaluate if any presented estrus provides another mount and ensure that said female was marked on the rump.
Postpartum management
Based on the dates of service, the possible dates of delivery were estimated, and at the time, the births of the lambs were attended. The number of lambs born per sheep, date and birth weight was recorded.
Response variables
Sheep in estrus (%): Sheep that showed acceptance to the male of the total of the treated sheep, expressed in percentage.
Start of estrus (h): Interval in hours between the last application of PGF2α and the external manifestations of estrus in the sheep.
Fertility (%): Sheep calving among the total of sheep served by natural service, expressed as a percentage.
Prolificacy: Number of lambs born between lambs born.
Fecundity: Total number of lambs born among the total of treated sheep.
Statistical analysis
The data was analyzed with the Statistical Analysis Systems® software (SAS Institute Inc, 2012). The start variable of estrus was analyzed with the Log-Rank survival curve method, by means of the LIFE TEST procedure and the comparison of means was analyzed with the Bonferroni method. The variables sheep in estrus, return of estrus and fertility were analyzed with the logistic regression model through the LOGISTIC procedure. The variables fecundity and prolificacy were analyzed by the confidence interval method for the difference of two Poisson rates.
RESULTAS AND DISCUSSION
Response to treatment
The male effect did not influence (P> 0.05) in the response to estrus in sheep synchronized with two doses of prostaglandins (Table 1). The response of the sheep in estrus in the present study is superior to that reported by Olivera-Muzante et al. (2013), who obtained 42.6 % estrus, but they are similar to what reported by Álvarez et al. (1994), who synchronized Pelibuey sheep with different doses of prostaglandins and observed 42 and 71 % response between treatments, until the seventh day after the end of treatment.
However, in post-treatment evaluations, they observed 100 % of estrus in estrus, and explained that in the first response there were possibly silent ovulations. Knight (1983) reported that the sheep presented silent ovulation six days after the introduction of the ram, and showed that 65 % of the sheep ovulate after 60 h of contact with the male; however, there are differences in the response according to the breed of sheep. In addition, it is possible that the transition from the anestrous season to the reproductive season is related to the low response to estrus, due to the fact that, at the beginning of the reproductive season, a high percentage of sheep have ovulations without the manifestation of estrus which is known as silent ovulations.
Table 1 Estrus response in sheep synchronized with two doses of prostaglandins (PGF2α) and male effect (ME)
| Variables | T1: PG | T2: PG+ME |
|---|---|---|
| n | 25 | 28 |
| Sheep in estrus (%) | 13 (52.0)a | 17 (60.7)a |
| Start of estrus (h) | 72.53 ± 6.05a | 45.13 ± 6.13b |
| Pregnant sheep | 12/25 (48.0)a | 15/28 (53.6)a |
| Lambed sheeps | 12/12 (100.0)a | 15/15 (100.0)a |
| Lambs born | 26 | 39 |
| Fertility | 12/25 (48.0)a | 15/28 (53.6)a |
| Prolificicacy | 26/12 (2.16)b | 39/15 (2.6)a |
| Fertility | 26/25 (1.04)b | 39/28 (1.40)a |
a, b: Valores con línea literal en la fila son diferentes (P <0.05).
In a study on Pelibuey ewes, Hernández et al. (2001) administered two doses of prostaglandins with an interval of eight days and observed that 64% of them presented failures in the regression of the corpus luteum after the second application, and estrus at 138 ± 13.7 h after treatment; concluded that estrus synchronization with this protocol based on the application of prostaglandins was not very efficient. In the induction of luteolysis, the corpus luteum is eliminated and the follicular phase is induced with ovulation (Abecia et al., 2012); however, in the present study it was not observed that the "male effect" induced reproductive activity in wool sheep, possibly due to faults in the luteal regression after the second application of prostaglandins. The protocols of estrus synchronization with two doses of prostaglandins in biostimulated sheep with "male effect" may differ according to the reproductive season; because the range of double doses can induce luteolysis and reduce treatment costs (Contreras-Solís et al., 2009); however, the frequency and intensity of the ram stimulus must be considered before or during estrus synchronization.
Start of estrus
The male effect influenced (P <0.05) at the onset of estrus in sheep synchronized with two doses of prostaglandins (Table 1). Using synchronization protocols with PGF2( without "male effect", it has been reported that estrus occurred at 36 ± 2.3 h (Letelier et al., 2011) and between 38.6 ± 0.5 and 51.6 ± 2.4 h (Fierro et al. ., 2013). These results differ with those obtained in biostimulated sheep with "male effect" in the present study. The start of estrus analyzed by survival curves (Figure 2), indicates that the sheep synchronized with two doses of prostaglandins and biostimulated with "male effect" began estrus sooner and more grouped, since, at 50 h later of the second application of prostaglandins, around 90 % of the sheep showed estrus, compared with those without "male effect", where the sheep obtained less response. The presence of the ram influences the onset of estrus, according to the drug applied in the synchronization of estrus, even when introducing vasectomized rams after applying the second dose of PGF2( to advance the onset of estrus (Ungerfeld, 2011).
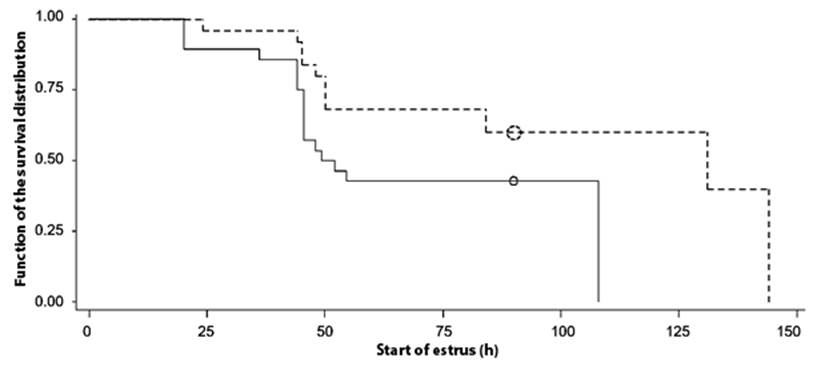
Figure 2 Survival curves for the start of estrus in sheep synchronized with two doses of prostaglandins (PGF2α) and male effect (ME)
The "male effect" is also capable of inducing ovulation, by the action of pheromones (Hawken and Martin, 2012), and in recent years, it is performed in hormonal treatments for the synchronization of estrus (to increase the secretion of the LH that produces the 'macho effect'); this combination is an effective alternative to reduce costs and improve efficiency in artificial insemination programs (Hawken et al., 2005); however, the response of the sheep and goats to the male effect depends on internal and external factors that operate in both sexes, such as female response variation to the presence of the males, the breed and the quality of the stimulus granted by males (Delgadillo et al., 2008).
Fierro et al. (2011) described that the interaction between the ovarian follicular development caused by the "male effect" in combination with the regression of the corpus luteum formed after the application of the two doses of prostaglandins, improves the grouping of the estrus, compared with the response of estrus synchronization without "male effect". Therefore, it is possible that the onset of estrus occurs in a shorter interval, if the dominant follicle is in the growth phase and therefore, in combination with the discharge of the GnRH secretion, where it perhaps favors the grouping of the estrus in the sheep. The synchronization with prostaglandins in the present investigation corresponded to the application of two doses with a separation interval of seven days, where possibly the sheep stimulated with "male effect" presented an active corpus luteum at the moment of applying the second dose of prostaglandins (Keisler, 2007).
Gestation and birth rate
No differences were found (P> 0.05) in the gestation and lambing rates of the sheep in the present study (table 1). The results for the gestation rate in the present study are lower than those reported by Álvarez et al. (1994) who obtained 85.7 % of births when synchronizing Pelibuey sheep with double injection of prostaglandins and providing service by natural one. On the contrary, Olivera-Muzante et al. (2013) reported 84.7 and 65.26 % return to estrus, and therefore obtained lower gestation percentage with a synchronization protocol with PGF2(. In addition, the technique of insemination also influences the percentage of pregnancy, such as 62 % of pregnant sheep synchronized with two doses of PGF2( and inseminated by laparoscopy (Fierro et al., 2011), higher than 42.6 % of pregnant sheep inseminated at fixed time (Olivera-Muzante et al., 2013).
Arroyo et al. (2009) mentioned that of the total of females biostimulated with "male effect", a high percentage ovulates between the first three to five days, because this stimulus causes an increase in the frequency of the release of the GnRH pulses and the LH, however, it is possible that the "male effect" in conjunction with the lysis of a corpus luteum could not induce fertile esters in the present study, which was observed in 48 and 53.6 % of fertility (P> 0.05). Some studies suggest that short hormonal treatments do not necessarily induce persistent follicles or result in low fertility (Vilariño et al., 2010).
Prolificacy and fertility
The male effect influenced (P <0.05) prolificacy and fecundity in sheep synchronized with two doses of prostaglandins (Table 1). The results of prolificacy and fecundity obtained in the present study surpass those reported by Fierro et al. (2011), who obtained prolificacy of 1.27 and 1.58 in synchronized sheep with double application of prostaglandins with respect to those with natural estrus, and reported an increase in follicular diameter with respect to that of sheep with natural estrus before ovulation. The increase in these parameters through the "male effect" is not very clear; however, it has been demonstrated that the sudden introduction of the male induces a rapid increase in the frequency and amplitude of the pulses of the plasma LH, this increase in the anterior pituitary activity stimulates a preovulatory peak of the LH which induces ovulation (Álvarez and Zarco, 2001).
Ungerfeld et al. (2005) reported that the introduction of the male with synchronized sheep with intravaginal sponges impregnated with MPA increased the follicular diameter (6 mm) with respect to those synchronized with sponges (5 mm). Additionally, there are results that indicate that the sudden introduction of the male improves the ovulatory rate in sheep (Hawken and Martin, 2012); however, these results have not been conclusive in this statement. Therefore, it is important to carry out more research on the biostimulation phenomena to know if it affects or benefits the reproductive efficiency of the females to establish more precise strategies in their use (Álvarez and Zarco, 2001).
The results suggest that the "male effect" induced a greater proportion of multiple births in sheep, a response that had been reported by Cognie et al. (1980), who showed that the ovulatory rate (number of ovulations per sheep) increased after the introduction of males in sheep herds, and this ovulatory rate increased in the second cycle. This may be due to the stimulation of GnRH secretion due to the "male effect", which is related to follicular growth and ovulation.
CONCLUSIONS
Biostimulation with "male effect" in sheep synchronized with a protocol based on two applications of prostaglandins with a seven-day interval, does not favor the response to estrus, but improves the onset of estrus in wool breeds.
The introduction of the ram on the fourth day after the first application of prostaglandins, does not improve the percentages of gestation, calving or fertility, but increases prolificacy and fecundity in wool sheep.
The timing of estrus based on two applications of prostaglandins in conjunction with the "male effect" is an alternative reproductive management in wool sheep during the reproductive season.











 texto en
texto en