Size scales in living organisms and nanotechnology
Life has been evolving for at least 3,800 million years. However, it was just after the development of nanosciences that the living cell has been recognized as a nanostructured world, a nanomachine. Overall, cells have sizes from 0.20.3 µm (in the smallest known bacteria) to 10-30 µm (in the case of most animal cells). Each cell is in turn organized in hundreds of subcellular compartments, inside which thousands of biomolecules are bumping around, following the rules of quantum mechanics (Goodsell, Olson, and Forli, 2020). Biomolecules have sizes within the nanoscale: 5-20 nm for proteins, 2 nm width in genetic strands, 5-7 nm thickness in cell membranes, and so on (Jeevanandam et al., 2018). Through nanotechnology, the possibility to control matter at such scales allows us a myriad of opportunities for addressing biomedical issues. A recent example is the development of RNA-based vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a platform based on lipid nanoparticles; thanks to nanotechnology, immunization with messenger RNA (mRNA) now constitutes a novel alternative in human vaccinology (Khurana, et al., 2021). Other promising developments include nanoparticles for drug delivery, bioimaging, sensing, and vaccines, as well as nanostructured materials for implants, organoids, theragnostics (i. e., a combination of therapy and diagnostics), and anti-microbial agents.
With nanotechnology, the possibility to obtain structures at specific sizes, forms and surface chemistries has led to materials that are highly compatible with cells and tissues. Nanostructures have been designed to deal with aggressive environments before reaching their target, or to respond only to site-specific conditions, such as acidic pH, temperature, and light or intracellular enzymes (Jeong et al., 2021; Wang et al., 2021). More and more biocompatible materials have been obtained, including metals, biodegradable polymers, ceramics, and self-assembled lipids (Figure 1). Some of these materials have electrical and optical properties, or surface areas leading to very high capabilities for drug encapsulation. Surface chemistries in nanomedicine can be designed to mimic cell surfaces. In animals, cells are surrounded by coatings allowing, for instance, stealthiness towards the reticuloendothelial system (RES) or the potential to crosstalk and be targeted towards other cells from distant anatomic sites (Castillo‐Armengol, Fajas, and López‐Mejía, 2019; W. Chen et al., 2018). The potential of such a fine communication machinery goes far beyond our comprehension, but it is well established that all these functions are triggered by cell surface molecules. Cells are surrounded by membranes made of amphipathic structures, mostly represented by glycerophospholipids (Figure 2). Also composed of proteins with apolar domains, cell membranes consist of lipid bilayers, where the polar motifs (which are protein, sugar, or nucleic acid in nature) act as the exchangers of information between the internal and external environment of cells. That is why the use of either isolated cell membranes, or cell membrane components for the decoration of nanoparticles stands as one of the most interesting approaches in nanomedicine. To date, membrane-coated nanoparticles have been obtained with the ability to migrate to specific body sites, or to show stealthiness towards the RES. Other biomimetic constructs have been designed so that, upon the addition of individual molecules, they interact with cell receptors at very high specificities (Ko et al., 2022; X. Huang et al., 2023). In the following sections, the main technolo- gies used for cell membrane biomimetism are presented.
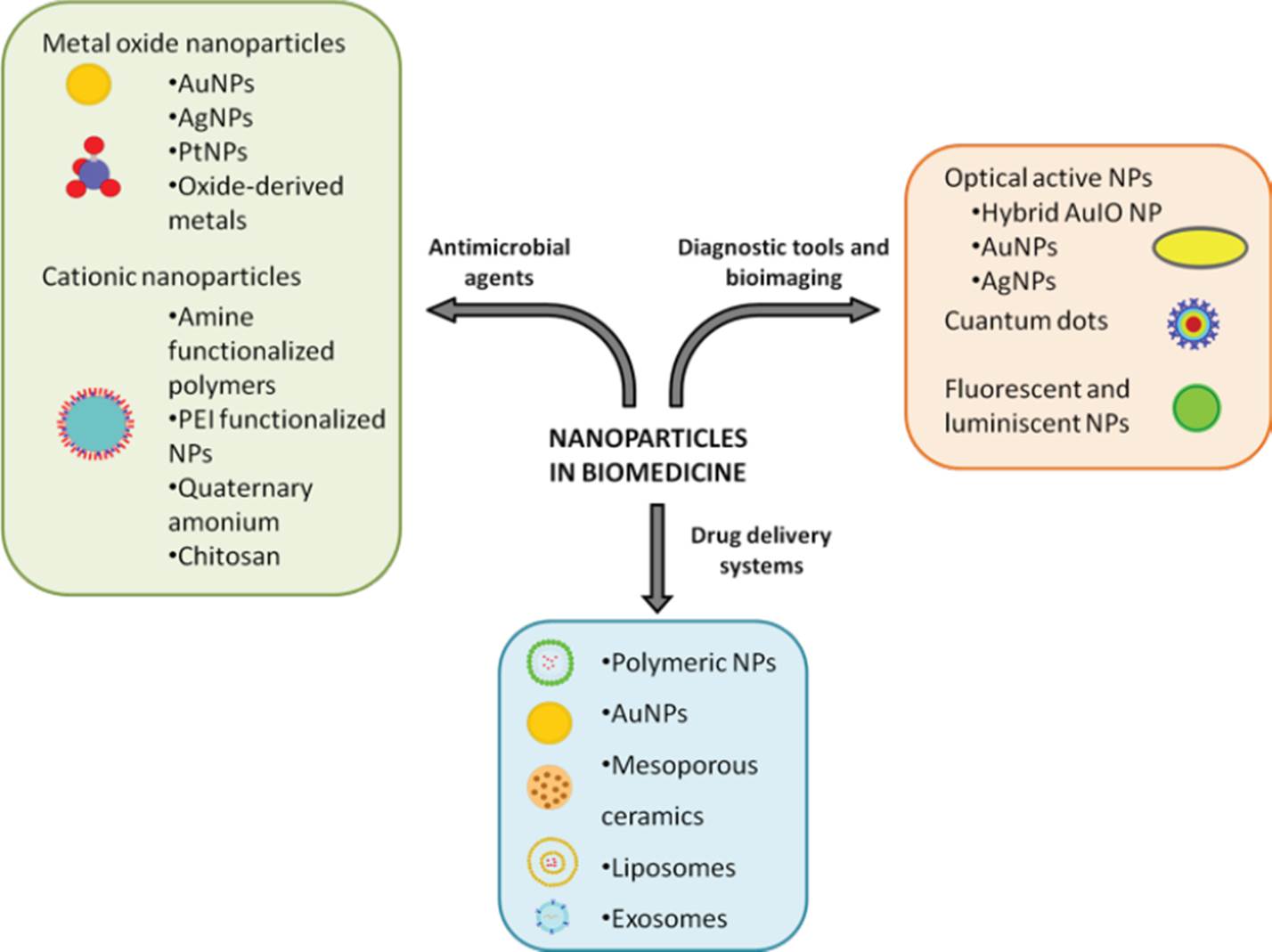
Different materials are suitable for their use in nanomedicine, allowing a selection of well-known chemical and textural features, according to specific medical purposes.
Source: Author’s elaboration.
Figure 1 Nanoparticles for therapeutic use.
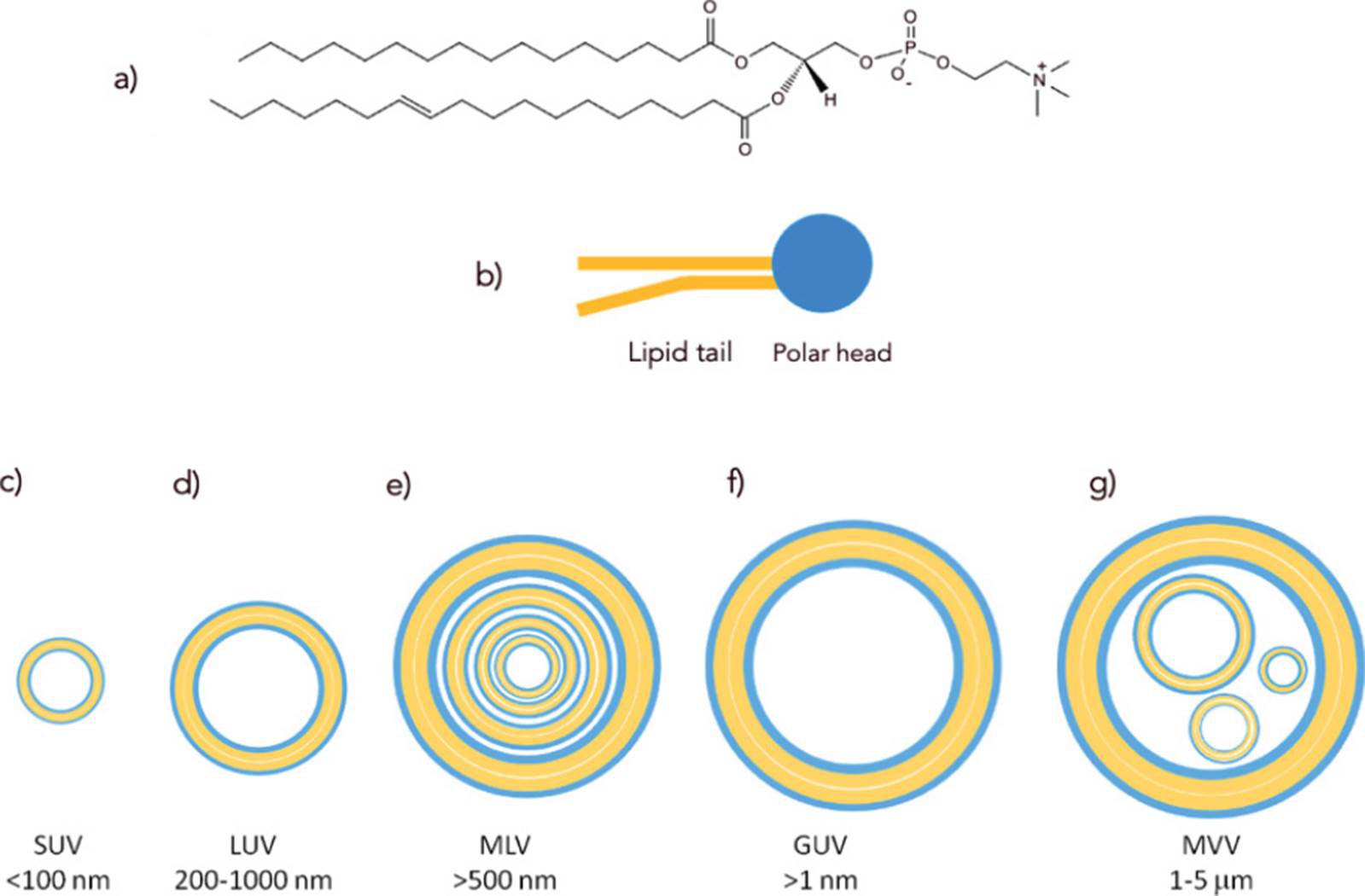
a) Structure of a typical glycerophospholipid, the major type of amphipatic lipid found in membranes; b) amphipathic lipids can be schematized by a polar head and a lipid tail; c) - g) liposomes are self-assemblies of amphipathic lipids, with one or more lamellae, according to their size and method of preparation. SUV, small unilamellar vesicle; LUV, large unilamellar vesicle; MLV, multilamellar vesicle; GUV, giant unilamellar vesicle; MVV, multivesicular vesicle.
Source: Author’s elaboration.
Figure 2 Liposomes are self-assemblies of amphipathic lipids.
Mimicking cell surfaces through supported lipid bilayers
The method of choice to mimic cell surfaces is the use of artificial membranes, also known as liposomes, which are self-assemblies of amphipathic lipids, the main components of lipid bilayers. In eukaryotes and bacteria, membranes are supramolecular structures where the major components are glycerophospholipids (phospholipids), a kind of amphipathic lipid consisting of a variable polar head, to which fatty acyl chains (with different structures) are attached through a phosphatidyl glycerol backbone (Figure 2). Each cell has a particular composition of phospholipids (Nelson, Cox, and Hoskins, 2021), which is also modulated according to cell location. The molecular composition of cell surfaces is complex and variable. However, the supramolecular nature of synthetic membranes allows the preparation of complex arrays, where lipid-anchored motifs can be easily displayed through self-assembled processes. Liposomes represented one of the first kinds of nanoparticles ever approved for therapeutic use in humans, as they have proved to be innocuous and biodegradable (Batty, Bachelder, and Ainslie, 2021); they can be engineered to have specific features such as biological recognition, permeability, and fluidity (Andra et al., 2022). Furthermore, their amphipathic nature make it possible to encapsulate drugs of distinct classes (Guimarães, Cavaco-Paulo, and Nogueira, 2021).
According to their structure, liposomes can be classified into unilamellar and multilamellar vesicles (Figure 2). Methods to synthesize liposomes include the use of organic solvents, mechanical procedures, or the removal of phospholipid/detergent micelle mixtures. The selection of the method can affect the final liposome structure (Alavi, Karimi, and Safaei, 2017). Typically, multilamellar vesicle preparation is achieved by dissolving amphipathic lipids such as egg lecithin, cholesterol, or phosphatidyl glycerol in an organic solvent. Then, a stream of nitrogen is used to generate a film and to remove traces of the solvent before adding an aqueous milieu (Giuliano et al., 2021). Finally, the treatment of multilamellar liposomes by ultrasonication, extrusion, freeze-thawing, or ethanol injection allows their rupture into unilamellar vesicles of submicrometric sizes. Other methodologies, such as electroformation, are used to obtain giant unilamellar liposomes, which have micrometric sizes and have been used as simplified models of cellular systems (Faizi et al., 2022). Thus, simple self-assembling processes are used to obtain liposomes of different sizes (Figure 2). In addition, unilamellar vesicles can be attached onto inorganic substrates to give arrays known as supported lipid bilayers (SLBs) (Figure 3).

Simple and biomimetic liposomes (above) or lipid bilayers from cells (below) can give rise to highly stable constructs, while interesting features of the core (high-loading capabilities, optical activities…) can be added.
Source: Author’s elaboration.
Figure 3 Synthetic and natural lipid bilayers can be stabilized through their attachment to other types of nanoparticles.
These hybrid arrays, made of inorganic and organic, lipid-based materials, usually give an increased stability to the bilayers (R. Zhang et al., 2021), along with properties given by the selected inorganic structure. To prepare supported-lipid bilayers, the interactions between the coat and the inorganic core is favorized through tailored chemistries. Lipid bilayers can be adhered to metal solid substrates using, for instance, thiolated lipids, which are known to strongly bind to metal surfaces such as gold (Pacchioni ,2019; Sakaguchi et al., 2017). Another strategy is the use of electrostatic interactions for binding positively or negatively charged lipid bilayers to oppositely charged materials (Valdemar-Aguilar et al., 2020). Multiple structures have been coated with lipid bilayers, including polymeric nanoparticles, porous silicon, mesoporous silica nanoparticles, hydroxyapatite and other calcium phosphates (Placente et al., 2018; J.-L. Huang, Chen, and Gao, 2018; Mukherjee et al., 2019). For drug/vaccine delivery purposes, the coating of nanoparticles using lipid bilayers give materials with features that include biocompatibility, biodegradability, easiness of drug encapsulation (through simple adsorption steps) and low cost. Owed with all these features, some of the most powerful drug carriers are membrane-coated mesoporous silica nanoparticles, also known as “protocells” (J. Liu et al., 2009). Based on this kind of constructs, complex biological phenomena have been mimicked by displaying bioactive molecules at the surface of mesoporous materials. Silica nanoparticles wrapped with liposomes may also include the ability to release biologically active substances without premature leakage, or the possibility to reconstitute crowded systems with distinct biomolecular components (J. Liu et al., 2009; Balouch et al., 2021).
Curiously, our group found that the use of liposomes to coat dense nanoparticles lowered the impact they have on cells concomitantly submitted to physical forces. A method widely explored to enhance the delivery of drugs in cells is the application of shock waves, which consist of mechanical waves associated with high pressure peaks (López-Marín et al., 2018). To increase the efficiency of drug delivery, shock waves are frequently combined with the use of soft material-based carriers, such as liposomes or lipid microbubbles. In contrast, dense materials have been known to heavily exacerbate the mortality of shock waves, thus banning the use of carriers such as mesoporous silica along with this physical method. In the search for optimal conditions for the genetic modification of human cells, Carrasco and colleagues found that, when coated with lipid bilayers, dense nanoparticles can be combined with shock wave treatments with no increase of cell mortality (Carrasco et al., 2016). This finding was interesting, since it demonstrated that particle surface modification through liposome coating may go far beyond the control of surface chemical interactions.
Being a bottom-up approach in nanotechnology, the preparation of liposome coats may take advantage of isolated membrane molecules to give specific functions. Nordlund and colleagues, for example, produced mesoporous silica particles coated with cytochrome c oxidase-containing membranes; then, they used the porous silica as a scaffold to demonstrate the redox-driven proton membrane exchange (Nordlund et al., 2009). Overall, supported lipidbilayers enable the exploitation of cell components that are commonly neglected due to their poor solubility in aqueous milieux; within synthetic membranes, hydrophobic structures can be displayed in water-stable colloids through non-covalent linkage, which may be critical as many surface molecules may act upon their trafficking into a cell receptor. In our group, mesoporous silica particles developed for vaccine purposes were coated with liposomes that included phosphatidyl-inositol-mannosides (PIMs), a bacterial lipid known to activate the innate immune system. Displayed within supported-lipid bilayers, PIMs were shown to bind to Toll-Like Receptor (TLR)-2, a macrophage cell surface molecule recognizing microbial patterns, thereby triggering an increase in phagocytic rates and the production of pro-inflammatory signals (Valdemar-Aguilar et al., 2020). Importantly, many naturally occurring amphipathic molecules, such as PIMs, are part of therapeutic formulations already in use for humans. PIMs are molecular components of Bacillus Calmette-Guérin (BCG), an attenuated bacterial strain used as a vaccine against tuberculosis. Although BCG is commonly used in neonates, its application has been proscribed in immunocompromised populations (J. Chen et al., 2023). Supported-lipid bilayers therefore offer the possibility to exploit isolated molecules endowed with interesting functions, like PIMs, in the context of non-living, safer biomedical systems.
Last but not least, lipid bilayers can be supported on planar surfaces to allow the preparation of highly biomimetic sensing platforms, including those based on micropatterned surfaces (Sut et al., 2021).
Isolated natural membranes and their use in nanotechnology
Membrane patches
Synthetic lipid bilayers are easy to prepare and highly modulable. However, many functions of cell membranes are multifactorial and depend on a set of proteins and glycans that are unique in each cell type and difficult to reproduce. In this regard, an alternative for biomimicry is the development of camouflages made with natural membranes. Cell membranes are complex and variable even within individual cells; however, there have been methodologies to isolate them since the early 1960s, when cell biologists started to separate cell organelles through differential and/or density gradient centrifugation.
During the last decade, multiple efforts have been dedicated to mimicking cell surface functions using natural membrane coatings. Membranes isolated from red blood cells have been used to enhance circulation times and reduce the clearance of nanoparticles (C.-M. J. Hu et al., 2011; Piao et al., 2014). Also, cell membranes from white blood cells have been used to produce hybrid particles that, in addition to avoiding their clearance by the RES, can communicate with cells by exerting targeting capabilities for improved delivery. The latter systems, also known as leukolike vectors or leukosomes (Molinaro et al., 2016), are usually prepared with membranes from macrophages or cytotoxic T-lymphocytes (Xuan et al., 2015; Rao et al., 2017; L. Zhang et al., 2017). Membranes from cancer cells, platelets and mesenchymal stem cells have also been exploited for targeting nanoparticles towards specific sites, either for therapeutic or imaging purposes (C.-M. J. Hu et al., 2015; Lai et al., 2015; Q. Hu and Gu, 2016; Min et al., 2019). Thus, the potential of natural membranes in nanotechnology is huge and multifaceted. However, there are technical challenges that must be solved for this approach to become a viable alternative in drug targeting. Recent reports have demonstrated, for instance, that the degree of coating in these constructs is low (< 50%), which strongly affects the fate of cell membrane-coated particles in the body (L. Liu et al., 2021).
Bacterial outer membrane vesicles
A strategy to generate safe and effective vaccines against bacteria is the employ- ment of outer membrane vesicles (OMVs). These particles are nano-sized structures formed spontaneously when portions of the outer membranes of Gram-negative bacteria separate from their envelope, encapsulating inside the periplasmic space. Because they contain highly immunogenic molecules, OMVs have a remarkable potential to induce immune responses (Ahmed et al., 2021). OMVs are 50 to 250 nm in diameter and are composed of phospholipids, proteins, and lipopolysaccharides (LPS) (Li et al., 2019). To obtain OMVs, the general protocol includes bacteria cultivation under specific stress conditions, removal of intact bacteria by low-speed centrifugation, and OMV isolation from culture filtrates through ultrafiltration, ultracentrifugation, and purification by density gradients (Klimentová, and Stulík, 2015). OMVs have diverse functions in bacteria, including their activity as decoys against bacteriophages, the elimination of extracellular harmful substances like antibiotics, biofilm formation (as OMVs contain biofilm matrix proteins), communication, and pathogenesis, due to the liberation of virulence factors that promote bacterial invasion to tissues (Furuyama, and Sircili, 2021). Due to these activities, OMVs have been proposed as nanotherapeutic agents. Firstly, they have been proposed to be used as drug nanocarriers since they can fuse with the membrane of target cells, have great stability and biocompatibility, and present long circulation times in the bloodstream. Also, OMVs can naturally load biomolecules, making them an interesting option for targeted delivery purposes. Moreover, OMVs may be modified through genetic engineering, mostly through permeabilizing techniques such as sonication and electroporation (Collins, and Brown, 2021), before their use in therapy, bioimaging and diagnosis (Xue, Wang, and Liu, 2022). As vaccines, OMVs are suitable due to their inherent adjuvant activity, leading to the activation of both the innate and acquired immune systems, along with their ability to activate cytotoxic T lymphocytes via cross-presentation by dendritic cells (Schetters et al., 2019). Finally, OMVs have been used to coat inorganic nanoparticles, allowing the production of highly immunogenic constructs (George et al., 2022).
Extracellular vesicles
Eukaryotic cells secrete different classes of phospholipid vesicles with a large set of roles in physiology and pathology. Although extracellular vesicles (EVs) were first evidenced in the late 1960s, a deeper comprehension of their biogenesis, complexity, and importance has only been obtained with the advancement of nanotechnology during the last three decades (Herrmann, Wood, and Fuhrmann, 2021). Some EVs are formed by budding from the cell membrane, whereas some others are pre-formed in the cytoplasm, then transported towards the extracellular space within multivesicular bodies (van Niel, D’Angelo, and Raposo, 2018) (Figure 4). Distinguishable by different compositions, the two types of EVs are commonly called ectosomes and exosomes, according to their origin (from budding processes or release by multivesicular bodies, respectively). One of the most interesting findings about EVs has been their role in cell-cell communication (Meldolesi, 2018). By recognizing a variety of specific cell markers and transporting biological mediators (notably, microRNAs), EVs may affect cell metabolisms at very distant tissues (Mathieu et al., 2019), an ability once thought to be limited to the neural and immunological systems. Therefore, due to their targeting capabilities and nanometric sizes, EVs are currently being explored as a powerful alternative for drug delivery (Kooijmans et al., 2012; García-Manrique et al., 2018).
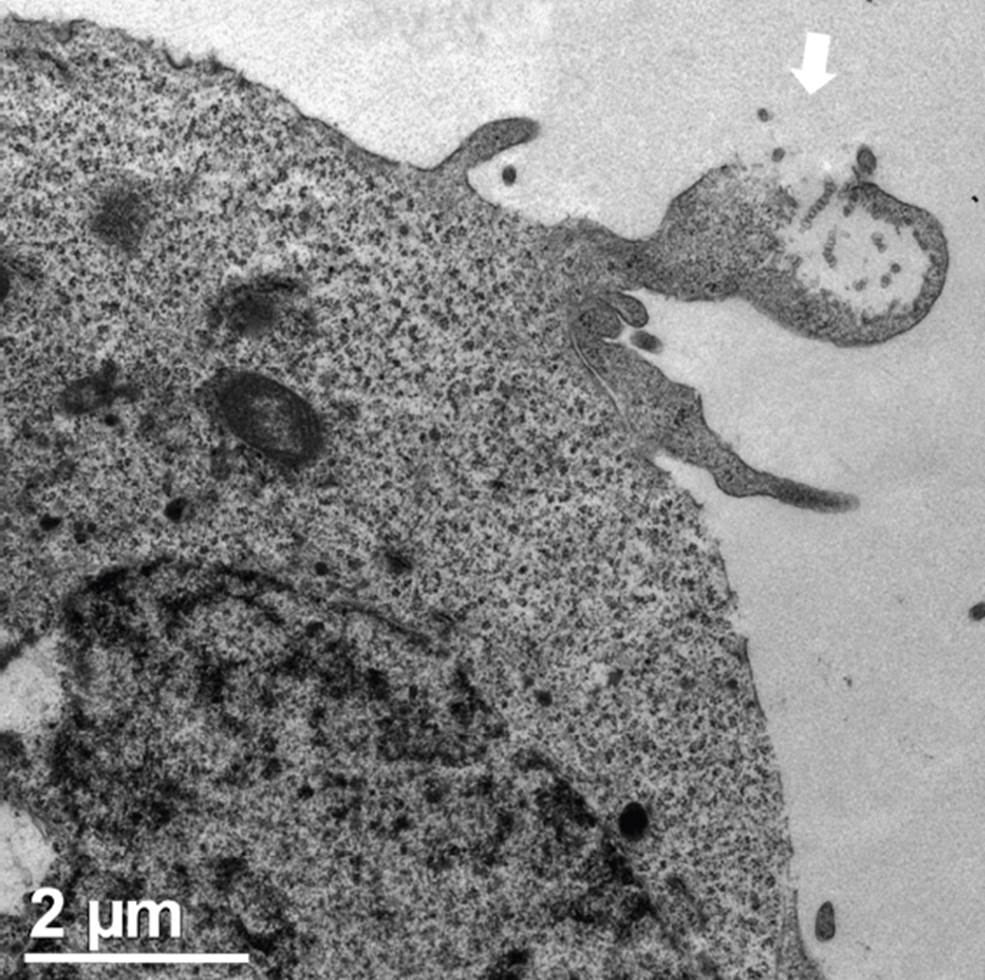
The white arrow points to exosomes liberated from a multivesicular body. Also visible are the nucleus and mitochondria.
Source: Author’s elaboration.
Figure 4 Transmission cell microscopy from a section of a HEK293 cell during the liberation of exosomes.
EVs are highly biocompatible, and their ability to change the behavior of cells in vivo has been demonstrated for over a decade (Lakhal, and Wood, 2011). Indeed, a set of EV developments are under clinical trials for the treatment of important medical conditions, including cancer, Alzheimer’s disease, cerebrovascular disorders and infectious diseases (Herrmann, Wood, and Fuhrmann, 2021). Interestingly, EVs obtained after the genetic manipulation of cells have been produced for the display of very specific molecules, such as targeting ligands or soluble mediators. Nevertheless, the multiple advantages EVs offer are very limited due to their low recovery from cell cultures, along with the high cost associated with their isolation (Li et al. 2019). A strategy to increase the production of EVs has been the application of treatments aimed to influence signaling pathways related to EV biogenesis (García-Manrique et al., 2018). Alternatively, the use of plant cells has been proposed as a practical, alternative source of EVs (Y. Liu et al., 2020), as well as the production of bioinspired exosome mimetics, also known as artificial EVs (García-Manrique et al., 2018). Although these semi-synthetic EVs do not have the high specificity of naturally occurring EVs, their feasibility through top-down bionanotechnology processes constitutes a promising option. Semi-synthetic EVs are produced from cells following either the modification of intravesicular/cell surface content or the encapsulation of exogenous molecules. Some of the strategies for this purpose are (i) the use of chemical reactions, (ii) the application of physical methods to transiently permeabilize preformed EVs, and (iii) the co-incubation of vesicles along with hydrophobic cargoes.
Finally, a disruptive approach based on top-down processes has been proposed for the production of EVs: the use of physical methods to induce the fragmentation of cell membranes, followed by spontaneous self-assembly to produce “EV mimetics”. Again, the capabilities of EV mimetics obtained this way are not comparable to those of naturally released exosomes. However, the possibility to obtain vesicles after the controlled expression of cell molecular determinants is promising. To date, the strategies used to produce EV mimetics include the extrusion of cells through polycarbonate membrane filters or microchannels and the use of silicon nitride blades in microfluidic devices (García-Manrique et al., 2018).
Concluding remarks
Most cellular functions depend on the interactions between the external and the internal cell environments, where cell membranes are major actors. Supported-lipid bilayers therefore represent one of the most powerful options in the field of drug delivery, with more and more possibilities given by the continuous increase of biomedical knowledge and growing advancements in cell technologies. To date, lipid bilayer-coated nanoparticles can be finely tailored for activities such as drug targeting, stealthiness, or immune modulation. However, important issues must be solved before these attractive assemblies can be fully exploited. Major limitations deal with the high yields needed for the preparation of supported-lipid bilayers at large scales. Further improvements are also required for controlling the ultrastructural features and the coating homogeneity of the assemblies. Only interdisciplinary efforts involving the different areas in nanomedicine may solve these drawbacks.











 nueva página del texto (beta)
nueva página del texto (beta)


