Introduction
Bronchopleural fistula (BPF) appears after pneumonectomy in up to 4.4 % of cases1. Open window thoracostomy (OWT) is carried out if conservative measures fail. They are often left to second intention spontaneous healing. Flaps are later used with obliterating purposes2.
These patients are complex to manage because the amount of available muscle is scarce, they have poor nutritional status, coexisting lung diseases, and other comorbidities.
Pectoralis major and minor muscles, serratus, intercostal, paraspinous, and rectus abdominis muscle flaps are used, but there are several drawbacks3. Free flaps are not the best option for such patients since they require complex procedures. Latissimus Dorsi, the major muscular source in this area, is split transversally through the pneumonectomy approach.
Conversely, the vertical musculocutaneous trapezius (VMCT) flap is left untouched and stays close to the problem, offering an elegant solution to this challenging scenario4.
Our experience with the VMCT flap in five cases is shown here.
Patients and methods
Surgical anatomy and technique
The blood supply of the VMCT flap is based on the deep branch of the transverse cervical artery. Between minor and major rhomboids, it gives a branch to the inferior part of the trapezius, which is the flap-feeding vessel. It travels caudally in a paravertebral axis along the trapezius muscle up to the 12th thoracic vertebrae. The flap design can even reach a lower level based on its random reserve5.
The lateral decubitus position allows joining both the thoracic window and the flap donor site in a single surgical field.
Debridement of the cavity is carefully done, and air leakages are identified if present.
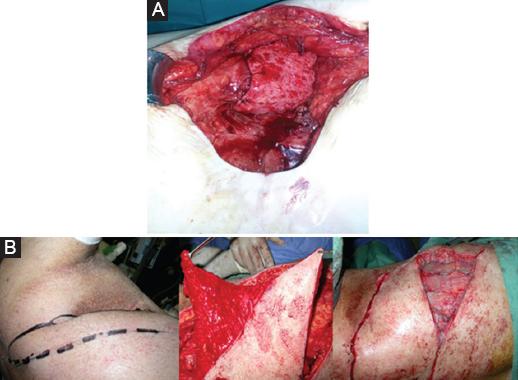
On the paravertebral ipsilateral flap donor area, the vessel and its route is identified with a Doppler ultrasound (Fig. 1). Once the vascular pedicle is identified, the whole flap is islanded. Then, a costal fenestration is done near the flap origin to allow for its intrathoracic passage6,7. This gateway into the thorax has to be in line with the transposition axis possibilities of the flap, allowing the rerouted flap to get a straight to the defect (Fig. 2) It is applied wrapping the bronchial stump after its closure if there is an air leak present (Fig. 3A). Other flaps may be used in combination if the cavity is still to be filled. We used a fasciocutaneous local flap (Fig. 3B).
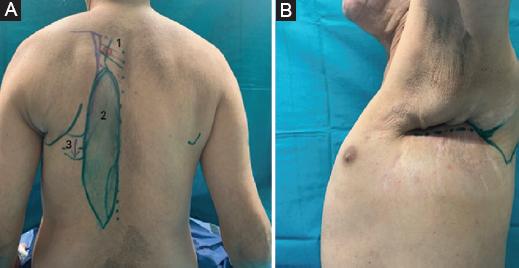
Figure 1 A: flap design: 1 Perforator nutrition vessel, 2 flap, 3 costal fenestration for rerouting. B: defect.
Clinical cases (Table 1)
Table 1 Patients' characteristics and data
| Case | Time from empyema to flaps in months | Cause of pneumonectomy | Adjuvant Radiotherapy | Adjuvant Chemotherapy | History of smoking | Infection of the BPF | Hospital stay in days after VMCT flap surgery | Number of total surgeries | Albumin level | % of pneumonectomy | Follow-up in months since VMCT flap |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 36 | Lung cancer | No | No | yes | Pseudomonas aeruginosa | 30 | 6 | 7.69 | 100 | 84 |
| 2 | 12 | tuberculosis | No | No | No | Mycobacterium abscesus | 23 | 6 | - | 66 | 78 |
| 3 | 21 | Lung cancer | No | Yes | Yes | - | 9 | 3 | 6.1 | 100 | 44 |
| 4 | 120 | Lung cancer | No | no | Yes | - | 10 | 3 | 5.2 | 66 | 43 |
| 5 | 36 | Lung cancer | Yes | Yes | Yes | Staphylococcus aureus | 21 | 3 | 6.9 | 100 | 12 |
| Case | SF-36 | Dysphagia | Dyspnea | QoL improved after surgery | Remaining wounds that require aditional care | Presence of pain in the thoracic area/Need of painkillers | O2 requirements before flap | O2 requirements after flap | Barthel Index | Current patient situation | |
| 1 | 48.61 | No | Yes. With minor efforts | Yes | No | Yes/ No | Yes | No | 100/100 | Stable | |
| 2 | 61 | Occasional | Yes. At rest | No | Yes. Requires serial dressings every 72h | Yes/ Every 8h | No | No | 80/100 | Chronic Mycobacteria infection with multiple admissions tot he hospital for antibiotic treatment, Chronic BPF and wound. | |
| 3 | 76.38 | No | Only walking long distances in hot weather | Yes | No | No/ No | Yes | No | 100/100 | Stable | |
| 4 | 74.30 | No | Only walking | Yes | No | Yes/ No | Yes | No | 90/100 | Stable | |
| 5 | 48.61 | No | Yes. With minor efforts | Yes | Yes. Requires serial dressings every 24h | No/ No | Yes. 24h a day | Yes. 22h a day | 100/100 | Chronic ulcer in donor site due to
previous RT. Worsening of the contralateral respiratory function |
|
BPF: bronchopleural fistula; QoL: quality of life; VMCT: vertical musculocutaneous trapezius.
CASE 1
A 47-year-old male diagnosed with a lung carcinoid tumor treated with right superior lobectomy in 2005 and right pneumonectomy in 2010. He developed a BPF which was initially managed with an OWT and later closed and sealed with a VMCT flap.
CASE 2
A 58-year-old woman with a history of tuberculosis and diagnosed with destroyed lung treated with a superior left lobectomy in 2012. She subsequently developed a BPF treated with an OWT. In 2013, a VMCT flap was performed. The BPF reappeared a month after surgery, and a second OWT was carried out.
CASE 3
A 71-year-old male with history an epidermoid lung cancer was treated with right pneumonectomy in 2014. He developed a BPF treated with OWT. In 2015, a VMCT flap was done.
Quality of life (QoL) and basic daily life activities (BDLA) assesment
QoL was assessed by the Spanish version of the SF-36. Each dimension of the questionnaire was assessed by questions in the survey, and item responses were coded and transformed into scores ranging from 0 (worst possible health state measured) to 100 (best possible health state)8. Barthel index was used to evaluate the BDLA and five additional questions that we considered relevant were asked: presence of (1) dyspnea, (2) dysphagia, (3) pain, (4) remaining wounds that required additional care, and (5) O2 requirements.
Results
In 80% of the patients, the BPF was sealed, the empyema residual cavity was filled and the patients' QoL improved.
Additional fasciocutaneous local flaps were used in 60% of the patients. Surgical time was under 3 h in all cases.
The average of the SF-36 score after flap surgery was 61.78. In 80% of the patients, QoL improved after surgery.
Dyspnea was present after surgery in all patients: 20% at rest, 40% with minor efforts, and 40% when walking long distances.
80% of the patients required oxygen before surgery and 20% after surgery.
All patients were independent for the BDLA after surgery with an average of the Barthel Index of 94/100.
Time average from empyema to flap in months was 45 (12-120); the cause of pneumonectomy was lung cancer in four cases (80%) and tuberculosis in one case (20%). Only one case had adjuvant radiotheraphy (RT) (20%), and two cases had adjuvant chemotheraphy (40%). Four cases had a history of smoking (80%). Time average of hospital stay in days after VMCT flap surgery was 18, 6 (9-30). Average of number of total surgeries was 4.2 (3-6). Average of percentage of pneumonectomy was 86, 4% (66-100%). Average of follow up in months since VMCT flap was 52, 2 (12-84).
Discussion and Conclusions
The VMCT flap represents the major non-affected musculocutaneous unit in the thoracic area after lung surgery. The rerouting of the flap through a costal fenestration allows a direct passage into the thorax, shortening the distance between the flap and the defect. Its dermal component offers a rigid matrix to form a seal over the bronchial stump. Its muscular component adds a good amount of vascularized tissue to the defect to fill and clean up the pleural cavity increasing antibiotic levels thanks to its rich vascular supply. No functional impairment has been described after its use. BPF can have an early or late pretentation, the most frequent being those that occur within the first 12 postoperative days9 Early BPF can be a vital emergency with high mortality, especially related to empyema and sepsis. Its treatment includes rethoracotomy, cavity drainage and OWT. There are different tecniques to perform OWT, and we describe them as follows10,11,12,13:
− Caglett's OWT consists in drainage of the pleural cavity by thoracostomy leaving the fistula open and daily changes of intracavitary bandage for a long period of time until its final closure with flaps.
− The Eloesser Flap OWT consists in making an “H” or “U” incision and creating a permanent drainage thoracic window towards the pleural space.
Other techniques used in the treatment of BPF were thoracoplasties which consisted in the resection of several costal arches to reduce the cavity which are no longer used. Endoscopic devices or VAC therapy are currently being studied.











 nova página do texto(beta)
nova página do texto(beta)