Introduction
The survival of the fasciocutaneous flaps is dependent on the adequate supply of oxygen from blood flow, throughout the entirety of the flap. The main challenge is in the distal areas of the flap, which suffer from a decreased supply, generating tissue ischemia1. Nitric oxide acts as a tissue protector by inhibiting aggregation of thrombocytes, leukocyte adhesion to the endothelium, eradicating free radicals, maintaining vascular permeability, regulating vascular tone, and stimulating the regeneration of vascular endothelium, among others2-7.
Dimethyl sulfoxide [CH3) 2SO] (DMSO) is a small polar molecule that contains sulfur at its center, 2 methyl groups, and oxygen at its tips. Due to its characteristics, it is one of the most common solvents, having the ability to penetrate membranes in skin, cells, and organelles. It can associate with various components including water, proteins, carbohydrates, nucleic acids, and ionic substances. Absorption capacity in humans has been demonstrated, and 5 min after topical application, it is detectable in blood8. With a peak at 4-8 h in serum after topical application or oral intake, elimination is registered 120 h after application. Its elimination route is through the kidneys9,10.
Various investigations have been carried out in rats regarding the attributes and different dosages of DMSO, seeking to quantify the optimal doses to obtain maximum benefit and minimum toxicity11. Among its qualities, it has been shown that, at low doses, it inhibits platelet aggregation, decreases pathological deposition of collagen in fibrotic tissue (without affecting the normal balance of collagen in healthy tissue)12, accelerates wound healing through the activation of fibroblast proliferation, mediated Akt/mTOR activation, leading to translation of proteins, such as collagen, and the secretion of TGF-β1 by fibroblasts, indirectly increases keratinocyte migration, stimulated by the increase imTGF-β113. It has a vasodilator effect by releasing a response similar to the release of histamine in the area of application. It is anti-inflammatory, by reducing the production of lymphocytes, neutrophils, IFN-γ, TNF-α, NF-KB, IL-8, IL-2, and prostaglandin E214. Analgesic, due to its ability to block C fibers of peripheral nerves15. It was identified to be effective when applied to pressure ulcers at an early stage, allowing a reduction in their torpid evolution, as well as analgesia of the affected area, reducing inflammatory signs such as redness, pain, heat, and increase in volume.
Nitroglycerin (NTG) increases prostacyclin synthesis by endothelial cells and acts as nitric oxide, whose main function is as a vasodilator and antithrombotic at the microvascular level. It acts both at the arterial and venous levels, its effect being more accentuated at the venous level16-18. It generates vascular dilation by relaxing smooth muscle, without altering pre- and post-capillary resistance19. In ointment form, it is safe, inexpensive, easy to apply, and fast-acting. In topical use, various studies have observed improvement of flaps that present ischemic signs. It has been used for multiple pathologies including coronary syndromes and anal fissures20,21.
Rohrich, in 1984, reported its topical use and effect on flaps in animal models, where he observed increased survival of the flap after the application of NTG22. Research has been carried out on its use in the area of breast reconstruction, taking into account that cutaneous necrosis after mastectomy is relatively frequent, occurring in between 2.5% and 60% of all patients. These studies showed improvement in the evolution and/or prevention of tissue ischemia. The latter presenting as a partial and full-thickness necrosis. Its use showed better effectiveness with serial application compared to a single dose. According to research in breast reconstruction, the dose that has proven to be safe and significant is 5.5 mg applied once a day every 48 h.
Methods
This project was approved by the Mexican Institute of Social Security animal care and ethical committee. Based on some articles published previously4,22,23, a blind, experimental study was conducted in 24 male Wistar rats, with a mean weight of 320 (286-376) grams.
The population was divided into 3 groups with 8 rats each one (n = 24):
Surgical technique
Anesthesia: 6.5% sodium pentobarbital was used, injected directly, intraperitoneally using a 1 mL syringe with a 25 Gg needle, at a dose of 50 mg/kg of weight.
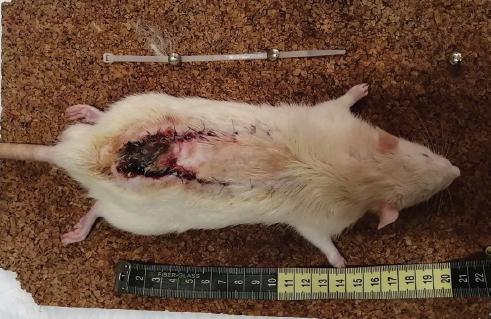
Once deep anesthesia had been verified by the absence of painful stimuli, a modified McFarlane flap23 for Wistar rats was designed with an indelible marker to be 3 cm × 10 cm with a cephalic base. Trichotomy, asepsis, and antisepsis of the area were performed with povidone-iodine.
Incision was made with a No. 15 scalpel blade and dissected with scissors until the entire flap was completed suprafascially. Subsequently, it was sutured with simple stitches using 4-0 nylon.
The ointment was applied by a researcher other than the main author, who carried out the statistical analysis and review of the results (blind). Topical application was carried out immediately after surgery and subsequently every 12 h for 5 days.
The survival and necrosis of the flaps were evaluated at 7 days using a transparent acetate sheet squared every 5 mm, to complete a total area of 3000 mm2, for subsequent analysis.
For the statistical analysis, measures of central tendency and dispersion were used, as well as the non-parametric Mann-Whitney's U-test, using the Statistical Package for the Social Sciences 10.0 program for windows (IBM; Armonk NY), taking as significant a p ≤ 0.05.
Results
A total of 24 rats were operated on in the 6-month period of this study, in the microsurgery laboratory of the UMAE 21 (High Specialty Medical Unit 21) of the IMSS (Mexican Institute of Social Security), in Monterrey NL.
The average weight was 320 g (limits 286-376 gr).
All the rats that participated in the study survived the observation period (7 days). The total area of the flap was 3000 mm2 in all experimental animals.
In the control group (petroleum jelly), mean necrosis of 1041.67 mm2 was observed, in Group I (NTG ointment) 616.67 mm2 and in Group 2 (DMSO) of 800 mm2 table 1.
Table 1 Flap survival and necrosis (in mm) by groups
| Group | n | Lower (mm2) | Higher (mm2) | Mean (mm2) | SD |
|---|---|---|---|---|---|
| Control | 8 | ||||
| Necrosis | 600 | 1400 | 1041.67 | 304.001 | |
| Healthy tissue | 1400 | 2400 | 1958.33 | 304.001 | |
| Nitroglycerin | 8 | ||||
| Necrosis | 350 | 850 | 616.67 | 183.485 | |
| Healthy tissue | 2150 | 2650 | 2383.33 | 183.485 | |
| DMSO* | 8 | ||||
| Necrosis | 450 | 1100 | 800 | 221.359 | |
| Healthy tissue | 1900 | 2550 | 2200 | 221.359 |
*DMSO: Dimethyl sulfoxide; SD: Standard deviation.
Using a non-parametric Mann–Whitney U-test analysis, a statistically significant p was obtained between the control group and 2% NTG ointment, both in the area of necrosis and in the healthy area (p = 0.026). In contrast, the comparison between DMSO and the control group (p = 0.180) and between both study groups (DMSO- NTG), with a p = 0.18, was not significant table 2.
Discussion
The vascularity of fasciocutaneous flaps has been extensively studied in recent decades; however, interest in them has increased exponentially since the 1987 work of Ian Taylor on skin territories and angiosomes24. Simultaneously, with the evolution of reconstructive microsurgery, multiple studies have been added about improvement in the survival of flaps. It is well known that the surgical technique at the time of flap dissection and the microvascular anastomosis are the most important prognostic factors for its success. However, there are many studies that look at adjuvant medications that can be used to increase local and systemic capillary vascularity1,2,4,6,11,15,17,19,20,22,23.
Two drugs widely known to be local and systemic vasodilators were used in our study: DMSO and NTG.
We found a difference in the means of necrosis between the control group (1041.67 mm2) and the NTG group (616.67 mm2). The results were favorable for 2% NTG ointment, with a statistically significant p compared to the control group (p = 0.026).
Since 1984, Rohrich et al.22 had already used NTG in experimental animals, also obtaining favorable results; however, unlike our study, he used axial flaps with a wider base (8 cm × 8 cm) based on the ventral inguinal vessels. In addition, some clinical studies in mastectomized patients have also shown a favorable response to NTG ointment17,19,20. Conversely, a study published by Nichter et al.21 concluded that NTG does not increase the survival of flaps in rats. However, this study used prolonged-release NTG skin patches (transderm-nitro 5), their main indication being for systemic absorption rather than local effect.
Unlike 2% NTG ointment, dimethyl sulfoxide did not reduce the risk of flap necrosis (p = 0.18), behaving similar to the animals in the control group, despite showing a decrease in the mean necrosis in relation to the control group (800 mm2 vs. 1041.67 mm2). We also found no statistically significant difference between the DMSO and NTG ointment groups (p = 0.18) (mean 800 mm2 vs. 616.67 mm2).
There are many studies in animal models that demonstrate a protective effect of DMSO in random flaps, based on the decrease in systemic pro-inflammatory factors (IFNg, TNF-α, NF-KB, IL-8, IL-2, and E2 prostaglandin). However, in many of them, DMSO is administered orally2,4 or injected subcutaneously11. In our study, we found that there is no such protective effect using topically applied DMSO ointment. Although flap survival improved in some of the rats, the difference compared to the control group and the NTG group was not statistically significant.
In clinical practice, the use of NTG ointment has been found useful to improve the survival of flaps5,8,17,19,20, these studies looked at flaps based on random circulation, where flap circulation is from capillaries and not from a main pedicle. In the case of axial microvascular flaps, where distress is mainly due to problems with the vascular pedicle, either at the level of the anastomosis or farther away due to torsion or kinking, the use of NTG ointment will not be useful, and the indication to reexamine the flap is the best salvage option.











 nueva página del texto (beta)
nueva página del texto (beta)