Introduction
Approximately 500,000 patients worldwide are diagnosed with cervical cancer every year. Cervical cancer is the fourth most common cause of cancer-related deaths in women and 85% of patients with cervical cancer live in low- and middle-income countries1.
The long-term presence of human papillomavirus (HPV) in the cervical epithelium is the most important etiological factor for cervical cancer2. More than 190 types of HPV have been defined, 14 of which have been shown to confera high risk of cervical cancer3. HPV types 16 and 18 are common carcinogenic genotypes in cervical cancer and are responsible for approximately 70% of cervical cancers. Together with HPV types 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68, these types constitute the high-risk (HR) group for cervical carcinoma4,5.
Performing a co-test, a combination of cytological examination (Pap test) with a polymerase chain reaction (PCR) test in which viral genotype is determined, is a very effective method in the early diagnosis of cervical cancer and therefore in reducing morbidity and mortality6. Primary prevention with vaccination programs in girls and young women and secondary prevention with HPV screening methods in older women forms the basis of cancer prevention strategies7.
Ten percentages of the country's population (therefore, approximately 8 million people) lives in the Southeastern Anatolia Region. This region, which is adjacent to the Middle East, is also the region with the highest percentage of the population comprising individuals under 18 years of age. To the best of our knowledge, there has not yet been a study on the prevalence of HPV in our region, in which vaccine type is most suitable for our region, and in which age groups should be included in the vaccination programs that are likely to be implemented in the coming years. This is an important public health issue. Thus, in this study, we aimed to determine the prevalence and genotype distribution of HPV in the women who arrived at our center, which provides care to a high volume patient population in the Southeastern Anatolia Region; compare the HPV types with cytological examination results; and demonstrate the effectiveness of the co-test.
Materials and methods
In this study, 13,300 cervical smear materials sent to our institution's pathology laboratory between 2015 and 2020 were evaluated retrospectively through the digital data system. Patients who had undergone hysterectomy and had inadequate results were excluded from the study. A total of 899 HPV-positive cases were included in the study. HPV-positive cases were divided into seven groups according to the age of the patient: under 19 years of age, 20-24, 25-29, 30-39, 40-49, 50-59, and over 60 years old.
Cytological diagnoses which were examined with SurePath liquid-based cervical cytology and HPV types determined by the PCR method were recorded. Cytological diagnoses were reported as non-diagnostic/unsatisfactory (ND), benign, atypical squamous cells of undetermined significance (ASC-US), and atypical squamous cells cannot exclude HSIL (ASC-H), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), squamous cell carcinoma (SCC), or atypical glandular cells (AGC) using the Bethesda Reporting System. The HPV genotypes were evaluated in six groups: (1) HPV type 16, (2) HPV type 18, (3) HPV type 16-18 association, (4) HPV type 16-HR association, (5) HPV Type 18-HR association, and (6) HPV HR (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). HPV genotyping studied by Roche Cobas 4800 equipment (Roche Diagnostics, Mannheim, Germany). This instrument performs automated DNA extraction and purification in cobas ×480, which is coupled to the cobas z480 thermocycler and analyzer for real-time PCR amplification and detection of target DNA sequences. This method detects HPV Type 16 and HPV type 18 and concurrently of a pool of other 12 HR-HPV genotypes (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). In our institution, samples examined with a single PCR. Statistical analysis was performed in the SPSS for Windows 10.0 statistical package program. Data are summarized as mean with range and percentage. Differences between groups were evaluated with one-way analysis of variance and categorical data were evaluated with Chi-square tests. Tests with p < 0.05 were considered statistically significant.
Results
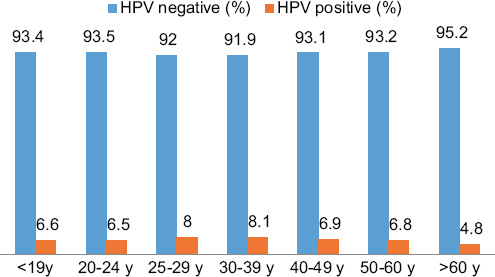
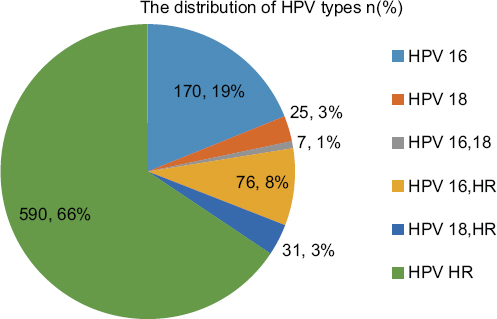
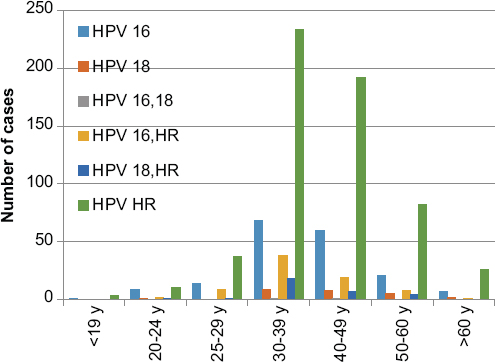
Eight hundred and ninety-nine (6.7%) of the cervical smear samples were positive for HPV DNA. The mean age of the cases in which HPV DNA positivity was observed was 41 years (range 15-78 years). For all groups of HPV types, the highest rates (8.1%) were observed in the 30-39 age range. HPV positivity among to age groups is shown in graph 1. HPV HR has the highest prevalence in all age groups. The distribution of HPV types is shown in graph 2. A statistically significant relationship was found between all age groups and HPV HR positivity (p < 0.05). The prevalence and types of HPV among to age groups are shown in graph 3. Among the six groups of HPV types, most of the cases (66%) belonged to group 6. The "benign" category was the most common (42%) diagnostic group for all HPV types in cytological examination. Cytological diagnosis categories and distribution of HPV types are shown in table 1.
Table 1 Distribution of human papillomavirus types among to categories of cytological diagnosis
| HPV types | Cytological diagnosis Number of cases (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ND | Benign | ASC-US | ASC-H | LSIL | HSIL | SCC | AGC | Total | |
| HPV 16 | 3 (1.8) | 55 (32.4) | 42 (24.7) | 2 (1.2) | 54 (31.8) | 9 (5.3) | 3 (1.8) | 2 (1.2) | 170 (100) |
| HPV 18 | 0 | 10 (40) | 9 (36) | 0 | 6 (24) | 0 | 0 | 0 | 25 (100) |
| HPV 16,18 | 0 | 3 (42.9) | 2 (28.6) | 0 | 2 (28.6) | 0 | 0 | 0 | 7 (100) |
| HPV 16, HR | 4 (5.3) | 21 (27.6) | 15 (19.7) | 1 (1.3) | 27 (35.5) | 7 (9.2) | 0 | 1 (1.3) | 76 (100) |
| HPV 18, HR | 0 | 17 (54.8) | 6 (19.4) | 0 | 8 (25.8) | 0 | 0 | 0 | 31 (100) |
| HPV HR | 16 (2.7) | 275 (46.6) | 171 (29) | 3 (0.5) | 101 (17.1) | 20 (3.4) | 1 (0.2) | 3 (0.5) | 590 (100) |
| Total | 23 (2.6) | 381 (42.4) | 245 (27.3) | 6 (0.7) | 198 (22) | 36 (4) | 4 (0.4) | 6 (0.7) | 899 (100) |
HPV: Human papilomavirus; ND: Non-diagnostic/unsatisfactory; ASC-US: Atypical squamous cells of undetermined significance; ASC-H: Atypical squamous cells can not exclude HSIL; LSIL: Low-grade squamous intraepithelial lesion; HSIL: High-grade squamous intraepithelial lesion; SCC: Squamous cell carcinoma; AGC: Atypical glandular cells.
When the relationship between HPV genotypes and diagnostic categories was evaluated, it was determined that the most common diagnostic category in the HPV HR group was the benign category (47%), followed by the ASC-US (29%), LSIL (17%), and HSIL (3%) categories (Figs. 1-3).
When we examined the cytological diagnosis categories, it was determined that 20 of the 36 cases in the HSIL category were in group 6, nine cases were in group 1, and seven cases were in group 4. There were four cervical carcinoma cases, three of which were positive for HPV 16, and one of which was positive for HPV HR. No statistically significant correlation between HPV types and diagnostic categories was observed. At the end of the 1st year, 64 patients, and at the end of the second year, 14 patients (66 in group 6, six in group 1, five in group 2, and three in group 4) had negative PCR tests.
Discussion
Cervical cancer is an important public health problem worldwide, and approximately 50% of cases are lethal8. Since the role of HPV in the development of cervical cancer has been revealed, screening and vaccination programs for HPV have been organized9. In epidemiological studies conducted in Europe and America, it has been shown that approximately 100% of patients with cervical cancer are positive for HPV10,11. It is accepted that the development of cervical cancer can be prevented to a large extent, especially with screening programs for patients in high risk group11. In the previous screening programs, HPV testing was requested if atypia was detected at at least the ASC-US level in the conventional Pap test; however, cotesting has recently been reported to be more effective. In addition, it has been shown that false positives and false negatives decreased significantly with the use of co-tests12,13. The American Society of Colposcopy and Cervical Pathology and the American College of Obstetricians and Gynecologists recommend screening every 5 years with co-testing in women aged 30-6514. However, the use of the Pap test alone as a screening method is still common worldwide. It has been reported that the specificity of the Pap test is higher than HPV DNA test and that it increases the diagnostic accuracy in HPV-positive patients15.
In four large European-centered randomized control studies, the diagnosis of cervical intraepithelial neoplasia (CIN) grade 3 or SCC was found to be significantly reduced in biopsy materials when screening with HPV or co-test according to cytology alone, in high-risk HPV-positive patients. It was determined that the co-test was 23-43% more sensitive than cytology in detecting high-grade CIN, but its specificity was significantly lower16-18. In our study, in which all cases were evaluated by co-test, it was determined that of the 899 HPV-positive cases, 530 were in the atypia category, and 369 were in the benign category according to the Pap test. This result supports the view that the HPV DNA test is more sensitive than cytological examination. The sensitivity of the HPV test is thought to be higher because viral DNA can be detected even in the early period of infection when the cytopathic effects of HPV have not yet occurred and because the results are less affected by external factors (removal technique, fixation, experience of the pathologist, etc.).
Determining the prevalence and HPV type of HPV-positive patients is important for planning vaccination and treatment strategies. In a study investigating the prevalence of HPV that collected data from 14 European countries, the lowest rate was found in Spain (2.2%) and the highest rate was in Denmark (22.8%)19. In a large-scale meta-analysis including studies conducted in different parts of the world, the worldwide prevalence of HPV was determined to be 10.4%, with the highest prevalence observed in West Africa (31.6%) and the lowest prevalence in Southwest Asia (6.2%)20 (Table 2). Different rates have been reported in studies conducted in Turkey. While the prevalence of HPV was reported to be 2% in the study by İnal et al. and 6% in the study by Özçelik et al.21,22, it was found to be 25% in a multicenter study by Dursun et al.23. In our study, the prevalence of HPV was determined to be 6.7%. The variability in the prevalence rates suggests that regional and socioeconomic factors are important parameters affecting the prevalence of HPV; notably, the rate was higher in hospitals that are reference centers. Notably, HPV vaccines are not included in the national vaccination program in our country. There are no published articles and official data on the HPV vaccination rate in our country. We think that in our region, which has a low socioeconomic level, the vaccination rate is low due to the fact that the vaccines are paid for and the awareness about the vaccine is low. Moreover, official data revealed that 69% of women in our country have never had a Pap test24. Although there is no national HPV vaccination program, the fact that the prevalence of HPV is below the world average suggests that sociodemographic characteristics affect the prevalence of HPV. Monogamy, early marriages, and the religiously conservative society may be factors.
Table 2 Worldwide distribution of human papillomavirus
| World region | Number of women tested | Number of women HPV-positive | HPV prevalence (%) |
|---|---|---|---|
| Total global estimate | 157 879 | 15.764 | 10.0 |
| Africaa | 6226 | 1429 | 22.9 |
| The Americasb | 40 399 | 6291 | 15.6 |
| Europec | 70 129 | 4649 | 6.6 |
| India | 19 164 | 1261 | 6.6 |
| Turkeyd | 13300 | 899 | 6.7 |
aEastern Africa, Northern Africa, Southern Africa and Western Africa.
bCentral America, South America and Northern America.
cEastern Europe, Northern Europe, Southern Europe and Western Europe.
dSoutheast of Turkey (Asia).
In the literature, HPV prevalence peaks in the 20-24 age groups in both developed and underdeveloped regions, with a second peak observed between 45 and 59 years of age in underdeveloped regions25. It has been reported that an HPV peak is seen in the 25-35 age group and decreases with age in some parts of Europe, Latin America and the Far East, while the prevalence of HPV increases with age in the underdeveloped regions of Asia and Africa26,27. In our study, it was observed that all HPV types peak in the 30-39 age group in our region, in contrast to what has been reported for European and Latin American countries. Statistical analysis revealed a significant correlation between age groups and HPV DNA positivity (p < 0.05). This could possibly be due to the social structure of the region prevented HPV from peaking at an early age. Therefore, we predict that extending the vaccination period up to the age of 30 would reduce the prevalence of HPV. Data on this topic can be obtained by conducting comprehensive vaccination studies that include long-term follow-up of the age groups of interest after vaccination.
It has been reported in the literature that HPV 16 and HPV 18 are detected in up to 70% of cervical cancers28. In the study by Zhou et al., the cases were divided into two groups, those from developed and those from underdeveloped regions, and the incidence of HPV 16 was 60-67% and 53-66%, respectively, while the incidence of HPV 18 was 10-13% and 11-15%, respectively, and other high-risk types were detected at a lower rate29. In a study of 248 cases of invasive cervical carcinoma from Turkey by Usubutun et al., the most common HPV types were determined to be, in the following order, HPV 16, 18, 45, 31, and 3330. Three of the four cervical carcinoma cases in our study were positive for HPV 16, and the fourth case was positive for HPV HR. In addition, the highest rates of the precancerous lesions HSIL and LSIL were observed in the HPV 16-HR association group. In accordance with the literature, it was determined that these cases should be followed closely. However, unlike the literature, the most common HPV type detected in the cases was HPV HR (66%).
It is known that approximately 90% of HPV-infected individuals become negative within 2 years, but high-risk types persist and progress to high-grade squamous intraepithelial lesions and cervical cancer28,29. Reflex HPV DNA testing is recommended in cases with cytological ASC-US. If HPV DNA testing is negative, an HPV DNA test should be performed after 1 year, since it is more sensitive than a Pap test30. The probability of the test results being negative during this period is high: A negative test is observed in approximately 50% of cases with HPV-positive28. In our study, it was determined that only 66 (11%) of HPV HR-positive cases, which were the most commonly observed type of HPV, had become negative by the end of the second year. We had a high proportion of women who did not return for subsequent screen. It is possible that they were followed up in health institutions close to the region where they lived and/or they were not followed up. Thus, the rate of negativity was lower than the rates reported in the literature.
This study has revealed that the prevalence of HPV in the southeast of Turkey is lower than the world average. In addition, it was determined that the most common HPV type in this region was HPV-HR and that HPV peaked at older ages. However, to obtain reliable results throughout the country, detailed studies evaluating large series from different regions are needed.











 nueva página del texto (beta)
nueva página del texto (beta)