Introduction
The prevalence of elderly patients in hemodialysis (HD) keeps growing as end-stage renal disease (ESRD) increases as well. It is estimated that the rate of patients over 65 years in HD grows more than 10% annually, only in the United States1, and about 11% of the HD patients in Australia are over 75-years-old2. Usually, these patients have shorter life expectancy, more comorbidities, and lower quality of life; although well established by Fistula First Initiative3 that the ideal vascular access (VA) in patients on HD is a native arteriovenous fistula (AVF), it seems to be not well-studied and a standardized practice in this age range.
There are reports of lower patency rates, lower maturation rates, and lower rates of AVF use in elderly patients4-7, probably because there are no standardized guidelines to determine which of these patients benefit from an AVF as a VA for HD. The primary and secondary patency rates reported at 1 year in the elderly range from 40% to 75% and 56% to 82%, respectively, some studies have published similar outcomes for both groups, against the concept of worst outcome for the elderly group8.
Due to the lack of clinical guidelines and recommendations in this specific population, the rates of AVF, arteriovenous grafts (AVG), and HD catheters are different from those reported in other populations and vary between countries and regions of the world; some groups reported that up to 75% of HD patients used catheters for this porpoise9. The 2018 ESVS Clinical Guidelines did not make a clear reference of decision making based on the age of the patients; they suggest permanent catheter in short life expectancy and mentioned a that a gap in evidence is "age should trigger access modality?"10. The 2019 KDOQI Clinical Practice Guideline for VA: 2019 Update does not make any specific recommendation in this group but recommends taking life expectancy and age of the patient to decide the best VA11.
The objective in this study was to evaluate the maturation and patency rates as well as complications in patients above 65 years that underwent a AVF creation and to compare them with our institutional experience with patients under this age. We hypothesize that with proper pre-operative planning, clinical outcomes should not be different among the age groups.
Materials and methods
We performed a retrospective analysis of the AVF created between 2011 and 2017 in patients with age > 65 years in our institution and compared them with patients under 65 years. We recorded demographic data, etiology of ESRD, and the reported patencies and complications associated to the AVF or the AVF creation. We used measures of central tendency for continuous variables. We used Chi-square test, with 95% of confidence interval and considering p value (< 0.05) as statistically significant for comparing frequencies. We used Kaplan-Meier analysis for patency rates during a mean follow-up period of 20 months and compared the patency rates between patients ≥ 65 years and younger. We used the maturation definition proposed by the 2018 ESVS guidelines, the rule of 6's: vein diameter of 6 mm, 6 mm of from skin to vein depth, and 600 mL/min flow10.
This study was approved by the Institutional Review Board of Clinical Research and follows their ethical standards in clinical research REF. 3548. Wavier of consent was obtained before enrolling subjects.
Results
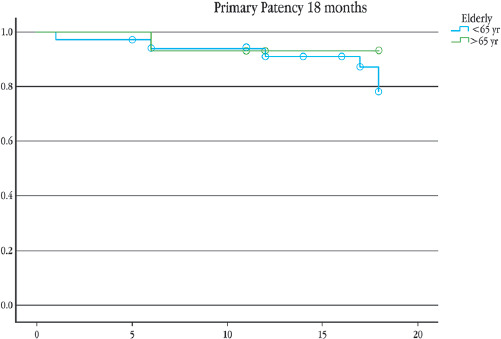
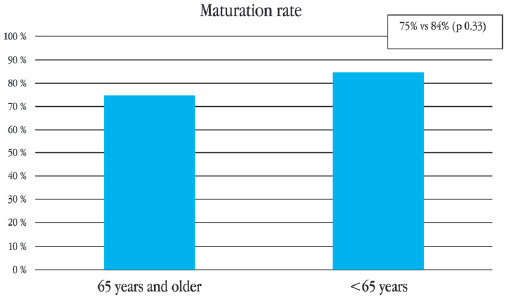
A total of 20 patients ≥ 65 years old underwent AVF creation for HD in our institution between 2011-2017; the mean age was 73 (SD ± 5.4) and 65% were man; comorbidities are exposed in table 1, only diabetes mellitus had statistical significance 60% for the elderly group compared with 33% (p = 0.05). From these AVF, 18 were brachiocephalic, and 2 were radiocephalic. No major complications reported. The primary patency (Fig. 1) at 6 and 12 months for the ≥ 65 years group was 93% and 86%, respectively, compared with 85% and 81% for the younger group (p = 0.77). The overall maturation rate was 75% compared with 84% (p = 0.33) of our patients < 65-years-old (Fig. 2). From the 15 patients with functional AVF, only one died < 2 years after the AVF creation, secondary to pneumonia, 1 year after AVF creation. The longest reported functionality of an AVF is 7 years.
Table 1 Comparison of patient's comorbidities and medication
| Variables | ≥ 65 years (n = 20) | < 65 years (n = 82) | p - value |
|---|---|---|---|
| Gender | F: 35% (7) M: 65% (13) |
F: 51. 2% (42) M: 48.8% (40) |
0.19 |
| Type 2 diabetes mellitus | 60% (12) | 32.9% (27) | 0.02* |
| Hypertension | 95% (19) | 95% (78) | 0.9 |
| Antiplatelet | 10% (2) | 2.5% (2) | 0.1 |
| Anticoagulation | 8.8% (7) | 0% | 0.5 |
Discussion
The reported maturation in our institution was 75%. We did not find a risk of non-maturation associated with the age of the patient, compared to a doubling of the risk in patients older than 65 years reported in the literature6. This association has been attributed to fewer adequate vessels in these patients; we have overcome this problem with a adequate pre-surgical vessel mapping, we have very strict protocols with the patients who are programmed to AVF creation no matter the age: it consist of pre-surgical venography (if more than one HD catheter has been used) and a bilateral mapping of the arm vessel, taking a minimum artery diameter as 2 mm and venous diameter of 3 mm. Our 1-year primary patency rate was 86% compared with 68 - 70% reported by Swindlehurst8. These results are contrary to a meta-analysis of 13 studies that reported a higher rate of fistula failure at 1 year for the ≥ 65 years or older group with OR 1.54; p = 0.001, and OR = 1.36; p = 0.01 at 2 years12. We did not see difference between the primary patency rates at 6 and 12 months from both age groups, 93% and 86%, respectively, compared with 85% and 81% (p = 0.77), we cannot establish age as a determining factor in the prognosis of the fistula. It is important to comment on our two patients over 80 years who underwent AVF creation; as of today, both are alive, one is 85 years old and the other one 92 years, both AVF is functioning. Although we found, significant difference between proportions of patients with type 2 DM in the groups, we do not think, is a factor in favor of maturation rates in the elderly group.
Secondary to the heterogeneity of the published information, there is not a consensus or guidelines for the use and type of VA for HD in elderly patients. Lok recently published their recommendations for selecting ideal VA for the elderly people undergoing HD, they concluded that this decision is not easy and depends on multiple factors. We cannot obsess with the idea of "Fistula First"13-16 in these patients, even though we report good maturation and patency rates, there are a lot of other factors we have to take in mind to make the decision. We like the concept adopted by Lok "patient first" strategy, the take in mind the patient's desires and preferences, as well as the expectations of the VA we will offer them; patient comorbidities and life expectancy must also be taken into account, taking in mind that a AVF takes an average of 90 days to maturate, as well as the risk of the chronic use of a central venous catheter for HD in these patients17. As commented in the 2018 ESVS access guidelines, this is still a gray area10.
The main flaw of our study is the low number of patients included but adds favorable data regarding outcomes in AVF creation in elderly patients; it is the largest report yet in Hispanic population; It is important to emphasize that we take all our potential AVF candidate to an exhaustive and meticulous pre-operative evaluation, more if they are 65 or older. Our patients underwent geriatric, cardiovascular, and anesthesiology evaluation before surgery, to establish life expectancy; once decided between the specialists that the AVF is the best option, we perform the vessel mapping and decide which configuration could the best, if no native vessels available, we decline the option for AVF; although there are some studies comparing AVF, grafts, or central venous catheters for the elderly18 that we are not comfortable using grafts due to the risk of complications an need of reintervention. Our institution minimum diameters are cephalic or basilic vein minimum of 3.0 mm without tourniquet compression, or 2.0 mm with an increase diameter of 0.5 mm minimum and the vein should have a non-sclerotic course through the arm; artery should be greater to 2.0 mm to be consider for AVF.
It is well known the differences in complications rates and mortality among countries, and ethnic groups, therefore, this study is important to understand the behavior of these procedures in a Mexican population. The weakness and limitations of this study include its retrospective nature, and that it is only one single center experience. Another detected flaw is that we only reported primary patency; unfortunately, we cannot offer rescue therapy for all the thrombosed patients, so the secondary patency is not reliable. We can use this as an example and start to formulate local and multinational guidelines for elderly patients in HD.
Conclusion
We reported higher maturation and patency rates than some of the published ones, and low complication rates, we even have one patient with 7 years functioning AVF. We conclude that AVF can and must be an option for selected patients with adequate cardiovascular reserve, fit for surgery and good life expectancy (over a year), and also to know the patient's preferences and expectancies from the AV for HD.











 nueva página del texto (beta)
nueva página del texto (beta)