Introduction
Neoadjuvant chemotherapy (NAC) is the first-line treatment for locally advanced and inoperable breast cancer (BC). Its main purpose is to achieve a pathological complete response (pCR). Achieving pCR after NAC is associated with improved long-term survival outcomes1-3.
NAC downstages the tumor and allows the de-escalation of breast and axillary surgery. It is increasingly used to allow the performance of breast-conserving surgery (BCS), thus avoiding mastectomy. Initially, biopsy-proven metastatic axillary lymph nodes may convert after NAC to become clinically negative and mean that axillary lymph node dissection (ALND) is not necessary3,4. NAC may provide a better prognosis while improving cosmetic outcomes and quality of life.
In luminal BC, NAC is less effective and has been reported to achieve lower pCR rates compared to the more aggressive subtypes3. Therefore, the decision to treat with NAC in patients with luminal BC remains controversial. Ki67, a nuclear protein associated with cell proliferation, is a well-established marker for predicting the outcome of patients with luminal BC5. Moreover, luminal tumors with high ki67 expression are reported to respond well to chemotherapy, possibly reflecting their high proliferative activity6.
In this study, we investigated the clinicopathological features of tumors and the effect of NAC on breast and axillary surgical strategy in luminal B, HER2(-) BC patients.
Materials and methods
Study design and patient selection
Patients diagnosed with breast cancer and treated at the University of Health Sciences, Turkey, Istanbul Haydarpasa Numune Training and Research Hospital, between January 2016 and December 2021 were retrospectively evaluated. This study was approved by the local ethic committee. Estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and ki67 statuses were determined by immunohistochemistry (IHC). For HER2, patients with IHC scores of 0, 1+, or 2+ and who did not show gene amplification by fluorescence in situ hybridization (FISH) were considered HER2(-). Based on the percentage of tumor cells with nuclear ki67 expression, tumors were classified as ki67-low (< 20%) or ki67-high (≥ 20%). The receiver operating characteristics (ROC) curve analysis was used to determine the cutoff point for ki67 levels.
All patients underwent pre-operative staging with ultrasound (US) and mammography (MMG). US-guided fine-needle aspiration cytology (FNAC) was routinely performed in patients with radiological suspected axillary lymph nodes (ALNs). Magnetic resonance imaging (MRI) was used to evaluate the extent of disease. Patients with ER +, PR ±, HER2(-), and ki67 ≥ 20% were designated as Luminal B, HER2(-) BC and who underwent NAC were included in this study.
Patients with bilateral breast cancer, distant metastatic disease, supraclavicular lymph node and/or intramammary lymph node metastases, and inflammatory breast cancer were excluded from the study.
Age, tumor size, multifocality, multicentricity, ALNs size, pathologic ALNs number, histopathological type, histological grade (HG), and nuclear grade (NG) were obtained.
In this study, seven patients whose ALN-FNAC result showed atypical cells were not included in the evaluation of axillary pCR. Of the 14 patients with complete pCR, only one patient had atypical cells in the ALN-FNAC result.
Evaluation of NAC response and outcome
To evaluate the contribution of NAC to surgical de-escalation, the type of estimated breast surgery that could have been performed if NAC could not be given was determined and compared with the actual surgery performed post-NAC. To decide on the pre-NAC estimated type of surgeries, pre-NAC US, MMG, and MRI findings were evaluated regardless of the pre- and post-operative histopathological results and chemotherapy responses of the patients. Pre-NAC multifocality and multicentricity were evaluated according to their radiological images.
Pre-NAC estimated breast surgery was categorized into two groups: Group 1 Mastectomy - Patients for whom curative results could not have been obtained with BCS because of tumor size, tumor extent, and breast volume; Group 2 BCS/Mastectomy - No obvious features, patients who could undergo BCS.
MD Anderson residual cancer burden (RCB) was reported to evaluate the chemotherapy response of the tumor and axilla. The RCB index categorizes patients with breast cancer into four groups (RCB 0-III) based on the level of residual disease after NAC. These categories are: RCB-0, pathologic complete response; RCB-I, minimal burden; RCB-II, moderate burden; and RCB-III, extensive burden.
Post-NAC tumor stage (ypT) and lymph node stage (ypN), residual tumor diameter, multifocality, multicentricity, surgical margin, number of ALNs removed, number of metastatic ALNs, and metastases diameter of ALNs were evaluated according to the post-operative histopathological results; pCR was defined as both the absence of invasive tumors in the resected breast specimens and the absence of metastases in the sampled lymph nodes (ypT0, ypT in situ and ypN0).
NAC
A standard taxane plus anthracycline-based regimen was used. Our treatment protocol was 4 cycles of doxorubicin (60 mg/m2) + cyclophosphamide (600 mg/m2) followed by 12 weeks of paclitaxel (80 mg/m2) or 4 cycles of docetaxel (75-100 mg/m2). Over 90% of patients were able to complete the treatment regimen. Adjuvant endocrine therapy with tamoxifen or aromatase inhibitor was started postoperatively, taking into account menopausal status.
Statistical method
The Statistical Package for the Social Sciences (SPSS), version 23, was used for statistical analysis (IBM Inc., Armonk, NY, USA). Descriptive statistics reported included mean ± standard deviation (SD), median and range or interquartile range, and frequency and proportions (%) were used when evaluating the study data. The conformity of the quantitative data to the normal distribution was tested by Kolmogorov-Smirnov, Shapiro-Wilk test, Skewness-Kurtosis test, and graphical evaluations. Mann-Whitney U-test was used for the comparison of two groups of data that did not show normal distribution. Kruskal-Wallis test was used for comparisons of groups of three or more that did not show normal distribution, and the Bonferroni Dunn test was used for pairwise comparisons. Pearson Chi-square test and Fisher's exact test were used to comparing qualitative data. Diagnostic screening tests including receiver operator characteristic (ROC) curve analysis were used to determine the cutoffs for parameters. Significance was evaluated at p < 0.05 level.
Results
Patient characteristics
During the study period, 128 patients were identified as complying with the inclusion criteria. The median age of the patients was 45 years (range: 18-77 years). The median ki67 proliferation index in the cohort was of our patients was 30% (range: 20-90%) (Table 1).
Table 1 Pre-NAC demographic, biochemical, radiological, and pathological characteristics of the patients
| (n = 128) | Characteristics | n | % |
|---|---|---|---|
| Age (years) | Median (range) | 45 (18-77) | |
| Ki67 (%) | Median (range) | 30 (20-90) | |
| Histological Grade | Grade 1 | 16 | 12.5 |
| Grade 2 | 63 | 49.2 | |
| Grade 3 | 49 | 38.3 | |
| Nuclear Grade | Grade 1 | 6 | 4.7 |
| Grade 2 | 55 | 43 | |
| Grade 3 | 67 | 52.3 | |
| Histopathological types | Ductal | 102 | 79.7 |
| Lobular | 5 | 3.9 | |
| Mixed-Others | 21 | 16.4 | |
| cT | 1 | 10 | 7.8 |
| 2 | 70 | 54.7 | |
| 3 | 15 | 11.7 | |
| 4 | 33 | 25.8 | |
| cN (n = 121) | 1 (mobile) | 113 | 93.4 |
| 2 (fixed) | 8 | 6.6 | |
| Tumor size (mm) | Median (range) | 30 (9-88) | |
| Number of metastatic ALNs (n = 121) | 1 | 48 | 39.7 |
| 2 | 18 | 14.9 | |
| 3 | 24 | 19.8 | |
| ≥ 4 | 31 | 25.6 | |
| Size of metastatic ALNs (mm) (n = 121) | Median (range) | 18 (6-53) | |
| Multicentricity | No | 87 | 68 |
| Yes | 41 | 32 | |
NAC: neoadjuvant chemotherapy; n: number; CT: clinic tumor stage; cN: clinic lymph node stage; mm: millimeter; ALN: axillary lymph node.
When the tumor stages were evaluated clinically, 10 patients (7.8%) were cT1. NAC was planned for these patients because they had biopsy-proven ALN metastases. Tumor size and the number of metastatic ALNs were evaluated according to the results of pre-NAC ultrasound (Table 1).
The median residual tumor diameter was 14 mm (range: 0-85) (Table 2). According to the pre-NAC MRI findings, the rate of multicentric or extensive disease was 32% (n = 41). The axillary pCR (ypN0/ypN0i) was 37.2% (n = 45). When the number of metastatic ALNs were evaluated according to the pre-NAC US results, one of them was biopsy-proven N+, 48 patients (39.7%) had one metastatic ALN, 18 patients (14.9%) had two ALNs, three ALNs in 24 patients (19.8%) had three ALNs, and 31 patients (25.6%) had four or more metastatic ALNs. The median number of removed ALNs was 12 (range: 1-30) (Table 2). When the pCR was evaluated by RCB classification, 10.9% of the patients (n = 14) had pCR and 16.4% (n = 21) RCB Class-I, while 42.2% (n = 54) were RCB Class-II, and 30.5% (n = 39) were RCB Class-III.
Table 2 Post-NAC surgical and pathological characteristics of the patients
| (n = 128) | Characteristics | n | % |
|---|---|---|---|
| RCB | 0 | 14 | 10.9 |
| I | 21 | 16.4 | |
| II | 54 | 42.2 | |
| III | 39 | 30.5 | |
| pCR | pCR | 14 | 10.9 |
| non-pCR | 114 | 89.1 | |
| ypT | ypT0 | 11 | 8.6 |
| ypTis | 7 | 5.5 | |
| ypT1mi | 3 | 2.3 | |
| ypT1a | 16 | 12.5 | |
| ypT1b | 16 | 12.5 | |
| ypT1c | 23 | 18 | |
| ypT2 | 37 | 28.9 | |
| ypT3 | 13 | 10.2 | |
| ypT4a | 0 | 0 | |
| ypT4b | 2 | 1.6 | |
| ypT0 (ypT0/ypTis) | 18 | 14.1 | |
| ypT+ | 110 | 85.9 | |
| ypN (n = 121) | ypN0/ypN0i | 45 | 37.2 |
| ypN1mi | 6 | 5 | |
| ypN1a | 35 | 28.9 | |
| ypN2a | 29 | 24 | |
| ypN3a | 6 | 5 | |
| ypN0 (ypN0/ypN0i) | 45 | 37.2 | |
| ypN+ | 76 | 62.8 | |
| Residual tumor diameter (mm) | Median (range) | 14 (0-85) | |
| pMultifocality | ypT0/ypTis | 18 | 14.1 |
| Unifocal | 79 | 61.7 | |
| Bifocal | 24 | 18.8 | |
| Multifocal | 7 | 5.5 | |
| pMulticentricity | No | 120 | 93.8 |
| Yes | 8 | 6.3 | |
| Surgical Margin (mm) | Median (range) | 8.5 (0.5-30) | |
| Pre-NAC estimated breast surgery | BCS/Mastectomy | 38 | 29.7 |
| Mastectomy | 90 | 70.3 | |
| Applied post-NAC breast surgery | NSM | 14 | 10.9 |
| SSM | 9 | 7 | |
| Mastectomy | 53 | 41.4 | |
| BCS | 52 | 40.6 | |
| Axillary surgery (n = 121) | SLNB | 38 | 31.4 |
| ALND | 28 | 23.1 | |
| ALND after SLNB | 55 | 45.5 | |
| Number of ALNs removed | Median (range) | 12 (1-30) | |
| Number of metastatic ALNs | Median (range) | 1 (0-19) | |
| Metastases diameter of ALNs (mm) | Median (range) | 4 (0-35) | |
NAC: neoadjuvant chemotherapy; n: number; RCB: residual cancer burden; pCR: pathological complete response; ypT: Tumor stage after neoadjuvant chemotherapy; ypTis: in situ; ypT1mi: micro; ypN: lymph node stage after neoadjuvant chemotherapy; ypN0i: isolated cell; ypNmi: micrometastasis; mm: millimeter; pMultifocality: pathological multifocality; pMulticentricity: pathological multicentricity; BCS: breast-conserving surgery; NSM: nipple-sparing mastectomy; SSM: skin-sparing mastectomy; SLNB: sentinel lymph node biopsy; ALND: axillary lymph node dissection; ALNs: axillary lymph nodes.
Initially, mastectomy was judged to be necessary in 90 patients (70.3%). The actual surgical procedures performed after NAC for these patients is shown in table 2 and figure 1.
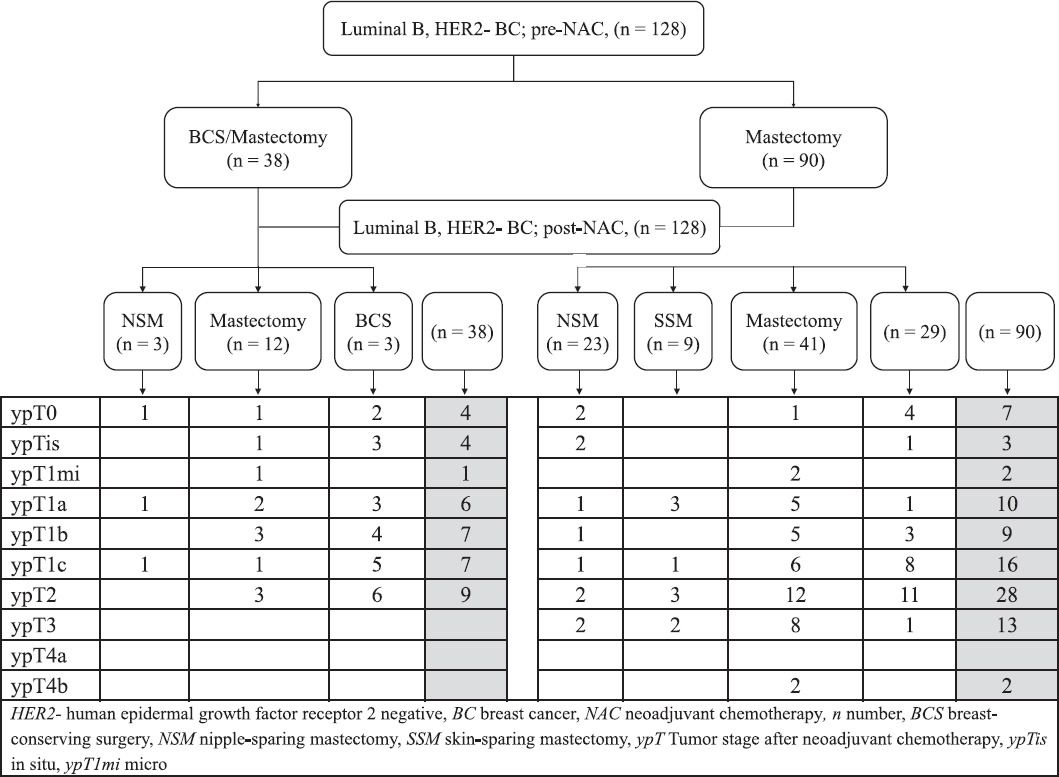
Figure 1 Evaluation of pre-neoadjuvant chemotherapy (NAC) estimated breast surgery, applied post-NAC breast surgery and tumor stage after NAC.
Of the 121 patients whose ALN-FNAC result was metastatic, 83 (68.6%) patients became eligible for SLNB after NAC. Since SLNB was positive in 45 of 83 (54.2%) patients, ALND was performed and the remaining 38 patients (31.4%) were spared ALND. It should be noted that ALND was not performed in two patients with micrometastatic SLNB results at their own request (Fig. 2).

Figure 2 Evaluation of applied post-neoadjuvant chemotherapy (NAC) axillary surgery and lymph node stage after NAC.
The ages of the patients in whom pCR did not occur were significantly older (p = 0.022) whereas the ki67 levels were significantly higher in patients with pCR (p = 0.036) (Table 3).
Table 3 Evaluation according to pathological complete response status
| n | Variables | pCR | p | |
|---|---|---|---|---|
| pCR | non-pCR | |||
| 14 | 114 | |||
| Age (years) | Median (Q1-Q3) | 41.5 (37.8-43.8) | 46 (38.8-56) | c0.022* |
| Ki67 (%) | Median (Q1-Q3) | 50 (25-70) | 30 (20-40) | c0.036* |
| Histological grade; n (%) | Grade 1 | 0 (0) | 16 (14) | b0.070 |
| Grade 2 | 5 (35.7) | 58 (50.9) | ||
| Grade 3 | 9 (64.3) | 40 (35.1) | ||
| Nuclear grade; n (%) | Grade 1 | 0 (0) | 6 (5.3) | b0.274 |
| Grade 2 | 4 (28.6) | 51 (44.7) | ||
| Grade 3 | 10 (71.4) | 57 (50) | ||
| Histopathological type, n (%) | IDC | 12 (85.7) | 90 (78.9) | a0.486 |
| ILC | 1 (7.1) | 4 (3.5) | ||
| Others | 1 (7.1) | 20 (17.5) | ||
| Multicentricity, n (%) | no | 11 (78.6) | 76 (66.7) | b0.368 |
| yes | 3 (21.4) | 38 (33.3) | ||
| Pre-NAC ALN number, n (%) | 1 | 7 (53.8) | 41 (38) | a0.232 |
| 2 | 2 (15.4) | 16 (14.8) | ||
| 3 | 0 (0) | 24 (22.2) | ||
| ≥ 4 | 4 (30.8) | 27 (25) | ||
aFisher Freeman Halton exact test.
bPearson Chi-squrare test.
cMann-Whitney U-test.
*p < 0.05.
pCR: pathological complete response; n: number; Q1: quartile 1; Q3: quartile 3; IDC: invasive ductal carcinoma; ILC: invasive lobular carcinoma; NAC: neoadjuvant chemotherapy; ALN: axillary lymph node.
Likewise, when ki67 levels and ypT status were compared, ki67 levels of ypT positive (ypT+) patients were found to be lower than those of ypT0. Based on this significant finding, cutoff points were calculated for ki67 levels according to pCR and ypT status. No statistically significant difference was found between ypN0 and ypN+ status and ki67 levels (Table 4).
Table 4 Evaluation of ki67 levels according to pCR, ypT and ypN status
| Variables | Parameter | n | Ki67 |
|---|---|---|---|
| pCR | |||
| pCR | Median (Q1-Q3) | 14 | 50 (25-70) |
| non-pCR | Median (Q1-Q3) | 114 | 30 (20-40) |
| p | 0.036* | ||
| ypT | |||
| ypT0 | Median (Q1-Q3) | 18 | 40 (28.8-62.5) |
| ypT+ | Median (Q1-Q3) | 110 | 30 (20-40) |
| p | 0.017* | ||
| ypN (n = 121) | |||
| ypN0 | Median (Q1-Q3) | 45 | 30 (20.5-50) |
| ypN+ | Median (Q1-Q3) | 76 | 30 (20-40) |
| p | 0.186 |
Mann-Whitney U Test.
*p < 0.05.
n: number; pCR: pathological complete response; Q1: quartile 1; Q3: quartile 3; ypT: tumor stage after neoadjuvant chemotherapy; ypN: lymph node stage after neoadjuvant chemotherapy.
According to the pCR status, the cutoff point ≤ 40% was determined for the ki67 levels. In cases with ki67 level of ≤ 40%, the risk of non-pCR was five times higher. According to the ypT status, the cutoff point ≤ 35% was determined for the ki-67 levels. In cases with ki-67 level of ≤ 35%, the risk of ypT+ was 3 times higher (Table 5 & Fig. 3A and B).
Table 5 Diagnostic screening tests and ROC curve analyses for ki67 levels according to pCR and ypT values, and the relationship between pCR and ypT status and ki67 levels (cut-off values)
| Variables | Diagnostic scan | ROC curve | p | |||
|---|---|---|---|---|---|---|
| Cut-off | Area | 95% confidence interval | ||||
| pCR | Ki67 | ≤ 40 | 0.669 | 0.518-0.820 | 0.040* | |
| ypT | Ki67 | ≤ 35 | 0.672 | 0.549-0.796 | 0.019* | |
| * p < 0.05 | ||||||
| Variables | pCR | non-pCR | >p | |||
| n | % | n | % | |||
| Ki67 | > 40 | 8 | 57.1 | 23 | 20.2 | 0.005** |
| ≤ 40 | 6 | 42.9 | 91 | 79.8 | ||
| Fisher's exact test. **p < 0.01 | ||||||
| Variables | ypT0/ypTis | ypT+ | p | |||
| n | % | n | % | |||
| Ki67 | > 35 | 11 | 61.1 | 38 | 34.5 | 0.032* |
| ≤ 35 | 7 | 38.9 | 72 | 65.5 | ||
Pearson Chi-square test.
*p < 0.05. ROC: receiver operating characteristics; pCR: pathological complete response; ypT: tumor stage after neoadjuvant chemotherapy; ypTis: in situ.
Discussion
In this study, the effect of NAC on the choice of a surgical method for breast and axilla in patients with Luminal B, HER2(-) BC was investigated. NAC has the benefit of downsizing the primary tumor, thereby making the feasibility of BCS more likely and may downstage axillary disease and spare the patients from ALND, especially in patients with HER2 positive and triple-negative BC. However, controversy remains regarding the benefit of neoadjuvant therapy in luminal BCs7. We found that patient age and ki67 level were the most significant variables when assessing NAC response in luminal B, HER-2 negative patients.
The ki67 status is not only of important prognostic value, it may also help make treatments for breast cancer more individualized. The clinical implication of ki67 levels is of critical importance in hormone receptor (HR) positive and HER2(-) tumors. In the study of Horimoto et al. Luminal, HER2(-) patients were evaluated and the pCR rate was 10% while the cutoff value for ki67 to distinguish pCR from non-pCR luminal breast cancer cases was 35%5. Other studies have also reported that ki67 cutoff values > 35% were found to predict pCR to NAC for the HR-positive and HER2(-) BC cases8,9. In our study, the cutoff value of ki67 for pCR was found to be 40%. According to ypT results, our cutoff value was found to be 35%. There was no significant association between ypN status and ki67 levels. In a study, the cutoff level of ki67 for ypN0 was found to be 30%, but they included all molecular subtypes in their study10.
In the prospective study of Mamtani et al., 85% of 155 biopsy-proven N+ patients with potential for downstaging became eligible for SLNB after NAC. The morbidity of ALND was avoided in 48% of cases, and this was in N+ patients, particularly in HER2 positive and TN BC. In ER+/HER2(-) patients, a higher rate of differential pCR was observed in the axilla than in the breast, with more than 20% of this group achieving nodal pCR11. In our study, 68.5% of patients became eligible for SLNB after NAC and more than 30% were spared ALND.
The most clearly established advantage of NAC is to convert patients who were initially ineligible for BCS. NAC has the potential benefit of reducing the size of the tumor, thus allowing BCS performance in patients initially requiring total mastectomy pre-NAC. The proportion of patients who underwent BCS after NAC ranged from 13% to 83%4. This wide range is probably due to different patient selection criteria in studies. Kim et al. found a BCS conversion rate of 16.3% in Luminal, HER2(-) BC, with no significant difference from rates in other subtypes4. According to pre-NAC MRI findings, the only option was mastectomy in 90 patients, but after NAC, 32% of these patients became eligible for BCS, exactly twice the rate reported by Kim et al. In our study, we evaluated not only BCS, but also patients who underwent reconstruction with NSM and SSM as a good cosmetic outcome. Among the patients who underwent mastectomy after NAC, three patients were ypT0 and 25 patients were ypT1, and BCS could be performed on these patients. However, the surgical preference of the patients and the fact that the marker was not placed at the beginning affected the post-NAC surgical treatment strategy.
In the study of King et al., it was reported that a surgical margin of no ink tumor is probably adequate for Stage I-III invasive breast cancer treated with NAC and BCS, in the absence of multiple scattered microscopic tumor foci12. In our study, pre-NAC these were 32% bifocal tumors and 25.8% multifocal by radiological assessment but post-NAC these proportions regressed to 18.8% bifocal and 5.5% multifocal histopathologically. The mean surgical margin of 110 patients with ypT+ was 11.46 mm. NAC facilitated safe surgical margins.
Our study has some limitations. It has a retrospective design. It was not always possible to determine whether a patient would be a candidate for BCS or total mastectomy before and after NAC. Patient preference after NAC also affected surgical decisions.











 nueva página del texto (beta)
nueva página del texto (beta)



