Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract (GIT)1-3. More frequently presented in the stomach1,3,4 and classified into localized and advanced or metastatic disease, which is directly related to the management2,5,6.
The standard management for localized GIST is the complete surgical resection1,2,4, and there are different approaches5,7,8. Since 2008, the laparoendoscopic cooperative surgery (LECS) has been proposed as a safe and effective alternative. We want to show our experience with LECS technique for the management of GIST and a literature review.
Materials and methods
A retrospective, cross-sectional study was carried out, which included patients with a diagnosis of localized GIST treated with LECS technique between January 2011 and December 2018. Before the procedure, each patient had an esophagogastroduodenoscopy (EGD) and a thoracoabdominal computed tomography (CT). The evaluated variables were location, tumor size, surgical time, intraoperative bleeding, conversion rate, number and time of complications, oral tolerance, hospital stay, reoperation rate, mortality, average of negative borders, and risk classification according to the pathology. These surgical procedures were performed by 3 laparoscopic surgeons. The protocol was implemented according to Helsinki's declaration of good clinical practice and approved by the Institution's Ethics Committee. No interest conflicts were declared.
Surgical technique
PATIENT PREPARATION
Initially, electrolyte status evaluation and fluid resuscitation were done, a Foley catheter was placed to quantify urinary output, and nasogastric tube was positioned for decompression with verification of proper function. Patients and their families were informed about surgical risks, possible complications, such as bleeding, infection, open conversion, and mortality.
All patients received prophylactic antibiotics, compression stockings, and intermittent pneumatic compression of lower limbs.
EQUIPMENT AND OPERATING ROOM PREPARATION
All patients were placed over the table in a supine position. Surgeon stands in American position at patient's left side and the first assistant stands at surgeon's right. The instrument was positioned at the first assistant's right.
With the open Hasson technique at the umbilical level, the creation of a pneumoperitoneum was performed, a 12 mm port was introduced with carbon dioxide maintaining an intra-abdominal pressure of 14 mmHg, a 30 degree laparoscope was advanced under direct vision, three ports were placed, one of 5 mm at the level of the right flank, one of 12 mm on the left flank, and one of 5 mm in the right upper quadrant. An intraoperative EGD was performed to confirm the location of the lesion (Fig. 1) and transilluminate it to limit the resection without sacrificing the oncological radicality of the surgery, greater curvature was dissected with a LigaSure-type surface sealing knife (Medtronic, Covidien, USA), to perform a limited gastric wedge resection endoscopy guided with a purple Endo GIA-type laparoscopic mechanical suture (Medtronic, Covidien, USA), hemostasis was reviewed. Surgical specimens (Fig. 2) were extracted in an endobag through an enlargement of the umbilical port protected with Alexis S type's surgical wound retractor (Applied Medical, USA). Cavity was aspirated. Ports were removed under direct vision, pneumoperitoneum was removed, and the aponeurosis was closed with simple interrupted sutures of PDS 0 points (Ethicon, Inc., Cincinnati, OH, USA) in the two 12 mm ports, skin was closed with Prolene 3-0 intradermal points (Ethicon, Inc., Cincinnati, OH, USA). Drains were not used.
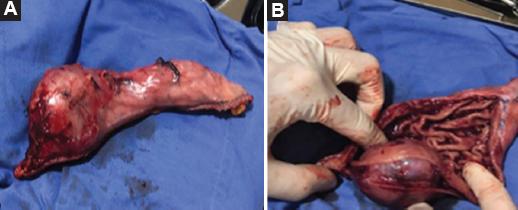
Figure 2 A: surgical specimen with oncological radicality surgery. B: surgical specimen extracted by laparoendoscopic technique.
If the patient presented GIST in the anterior gastric wall near to the esophagogastric junction or in the lesser curvature, an anterior total gastric wall resection with primary closure was performed using interrupted PDS 3-0 plus 330 fundoplication wrap. However, if the GIST was localized in the esophagogastric junction or the posterior gastric wall at the lesser curvature, a subtotal or total gastrectomy was performed with a Roux in Y reconstruction.
Post-operative management consisted in orally intake 24 h after the procedure with liquid diet and regular diet the next day, multimodal analgesia was used, and in the absence of medical complications, patients were discharged.
Statistical analysis
A descriptive analysis of the data was done, prospectively in Microsoft Excel databases and using Statistical Package for the Social Sciences 1 version 22.0. Continuous variables were analyzed by ranges. Variables are summarized using median, minimum, and maximum value and percentages.
Results
Among January 2011 and 2018, 21 patients with localized gastric GIST were operated using the LECS technique. Mean age of patients was 68.9 years and the male-female ratio 3:1, more demographic characteristics are described in table 1. The most frequent location was the greater curvature (50%) followed by the esophagogastric junction (20%) and the gastric fundus, the lesser curvature, and the antrum 10% each one. The average size of the tumors was 3.3 cm and the operating time was 98.5 min with negative borders in 100%. Pathological classification was low risk in 80%, intermediate risk in 15%, and high risk in 5%. Patients had an average of 30.7 cc bleeding. ICU admission and reoperation were 2.8% each. None of the patients required post-operative drainage. Orally intake was achieved in the first 24 h all patients, hospital stay averaged was 1.9 days, and mortality was 0% with no complications at the follow-up.
Discussion
GISTs are part of soft-tissue sarcomas4 and are the most common mesenchymal neoplasm of the GIT1,2,4,5,6,9. Its estimated incidence is 1/100,000/year2. GISTs are more common in men and their average age of presentation is between the 4th and 6th decade, in our study, the mean age of patients was 68.9 years old and male-female rate 3:1. In child population GISTs are rare, and are more common in girls1,2,6. Between 10% and 30% of GISTs have a malignant course5. About 80% of our patients had low pathological classification risk, 15% intermediate risk, and 5% high risk. Regarding the location, its distribution on the GIT is variable (Table 2)1,6,10. Our cohort reported the greater curvature of the stomach as the most frequent, however, literature shows differences, as Qiu et al. study where stomach's middle third was the main location11, Matsuda et al. upper third12, and Okumura et al. stomach's body13.
Table 2 Location frequency of GIST
| Location | Frequency (%) |
|---|---|
| Stomach | 60 |
| Jejunum and ileum | 30 |
| Duodenum | 5 |
| Rectum | 1 2 |
| Esophagus | < 1 |
| Epiploon, mesentery, retroperitoneum | Rare |
GIST: gastrointestinal stromal tumor.
Standard treatment for localized GISTs is surgical resection1-6,14, lymphadenectomy is not advised15, and surgical goals are to achieve R0 resection, avoid tumor rupture, and preserve organ function16,17. Surgical approach can be done by open surgery, laparoscopy, endoscopy, or laparoendoscopic cooperative surgery obtaining different results according to the chosen management10,14,18.
Since 2008, a group of surgeons and doctors combined these two techniques and the new approach called LECS emerged18. Using this technique, oncological margins are guaranteed, excessive resection of the gastric wall is reduced, no mortality has been reported and decreases the incidence of postoperative complications15,18-20. During this procedure, an intentional opening of the gastric wall must be made, which is why the possibility of tumor spreading has been described as the main limitation14,17-19. None of the studies reviewed for this publication (Table 3) had this complication presented. To overcome this problem, procedures such as inverted LECS have been developed18.
Table 3 Comparison of GIST management studies with LECS
| Author/year | Qiu et al./2013 | Matsuda et al./2016 | Kikuchi et al./2017 | Okumura et al./2017 | Pulido et al./2021 |
|---|---|---|---|---|---|
| Patients | 69 | 100 | 10 | 28 | 21 |
| Average age (years) | 57.6 | 59.8 | 62 | 67.8 | 68. 9 |
| Most frequent location | Stomach middle third | Stomach's upper third | Not reported | Stomach's body | Greater curvature |
| Average size (cm) | 2.8 | 3.09 | 2.4 | 3.3 | 3.3 |
| Surgical time (min) | 86.3 | 174.3 | 253 | 126 | |
| Bleeding (ml) | 31.4 | 16.3 | 18 | 10 | 30.7 |
| Orally tolerance (h) | 84 | 34 | Not reported | 72 | 24 |
| Complications | Suture line leak (1). Gastrointestinal bleeding (1) | Leak (1). Stenosis (2). Post operative bleeding (1). | Intra abdominal abscess (1) | None. | Intraoperative bleeding (1). |
| Hospital stay (days) | 4.6 | 8.4 | 9.2 | 7 | 1.9 |
| Negative borders | 100% | 100% | 100% | 100% | 100% |
| Mortality | 0% | 0% | 0% | 0% | 0% |
| Conversion | 0% | 5% | 0% | 0% | 2.8% |
GIST: gastrointestinal stromal tumor; LECS: laparoendoscopic cooperative surgery.
According to the clinic up to 69% of patients are asymptomatic as our study where all patients were asymptomatic6, however, they may present nonspecific symptoms such as epigastric pain, vomit, weight loss, abdominal guarding, gastrointestinal bleeding, nausea, anorexia, early satiety feeling2,21 intra-abdominal hemorrhage, and, in rare cases, acute abdomen1,9 (especially in patients with small bowel GIST)9. For that reason, it is usually an incidental finding during an endoscopy, diagnostic images, or surgery1,6. GIST > 2 cm usually is symptomatic7.
Diagnosis begins with the radiological examination, EGD has a sensibility that varies between 89% and 90% and its specificity between 29% and 64%. Ultrasound has 89% of sensibility and 70% of specificity22, and shows hypoechoic masses capable of displacing nearby structures. CT and magnetic resonance imaging allow to obtain better information about location and size10. Ultrasound is the gold standard because it makes both macroscopic and microscopic diagnosis by allowing biopsy to be taken7,9. In our study, all patients had EGD and CT before the procedure. Diagnosis confirmation is only possible through histology and immunohistochemistry of the lesion. [10] Within the immunohistochemical profile, the most common marker is the receptor tyrosine kinase CD117 (KIT)1,2,5-7,10,17,23.
Dimensions can vary from millimeters to more than 35 cm. Qiu et al. included 69 patients in their study, reporting an average tumor size of 2.8 cm11, smaller than ours that was 3.3 cm. Regarding their morphology, GISTs can be masses on GIT wall or have a polypoid appearance that predominates toward the mucosa or serosa2. They metastasize mainly to the liver and peritoneum. Lymph node metastasis is rare1,5,9.
A study published in 2017 where LECS technique was used for the treatment of gastric GIST; the average surgical time was 126 min13, another study published in the same year reported 253 min as the average surgical time24, contrasted with us, which was 98.5 min.
Bleeding reported in studies ranged between 10 and 31.4 mL (Table 3); ours was close to the upper limit (30.7 mL). Regarding the post-operative term, our patients had the shortest beginning of orally intake within the first 24 h, compared to studies where an average up to 84 h was reported11. Our cohort had the shortest hospital stay (< 48 h), in contrast with studies such as Kikuchi et al., where hospital stay was 9.2 days24. Last two variables mentioned can be the subject of future studies to evaluate if the conditions that allowed them can be reproduced.
Complications generally have a low incidence in patients managed with LECS technique11,20. Matsuda's study, with a cohort of 100 patients, reported only 4% of complications12. There are even studies such as Okumura's where zero complications were reported13. In our study, there was only one complication (gastrointestinal bleeding that required conversion in 4.7%). Studies reviewed follow a pattern similar as ours with a report of 100% negative margins and 0% mortality, confirming efficacy, and safety of this technique (Table 3).
This study is limited because it did not have a control group and the follow-up of the patients was too short. Making necessary new research studies with short- and long-term follow-up to obtain knowledge about outcome of patients with gastric GIST managed through LECS technique.
Conclusions
The LECS technique has demonstrated to be a viable, safe, and effective technique for the management of gastric GIST's. Showing superiority in organ function preservation and in the range of oncological margins. Prospective studies are necessary to obtain knowledge about the outcome of patients managed through LECS technique.











 text new page (beta)
text new page (beta)



