Introduction
The T2DM continues focusing a major attention in the biomedical research not only in the medical aspects but also in the surgical facets. The resolution of T2DM after bariatric or metabolic surgical processes is an clinical eidence. Many hypotheses have been reported to explain the physiological elements, which are involved in this biological and medical reality1.
The mechanisms related to the reversion of T2DM have included many theoretical ideas. The classical theories of distal and proximal described by Rubino2 are actually reformulated with new contributions3,4. The changes in gastroenteral hormones released after bariatric surgeries have also invoked with clear effectors. GLP-1, PYY, GIP, or Ghrelin have been related to several physiological consequences on glycemic control5,6. Other important aspects as microbiota, biliary salts, or the main control of central nervous system are not excluded of this multifunctional complex system3,7.
Thus, the enteral hormones have not been related not only to the glycemic control but to the consequences on pancreas cellularity8. The basal and modified levels of these marked hormones have been broadly studied on the glycemic control and the correlation with T2DM improve. Actually, some hormones have been signed too as effector in these cellular changes. Thus, after bariatric surgeries, the endocrine pancreas cellularity could be implied in the islet plasticity as in major metabolic circumstances.
Similar cellular changes have been reported in endocrine pancreatic cells after bariatric surgeries in healthy animal models. These reports included the histomorphometric analysis of the pancreatic islets with significant differences according to the surgical aggression on the digestive tube9,10.
Now, our aim is to study the histological consequences on the islet conformation after a severe malabsorptive surgery in diabetic rats. This morphometric analysis could be related to the relative cellular changes, according to dedifferentiation processes. To this, we used diabetic Goto-Kakizaki (GK) rats, to observe the morphometric changes in an atopic islet. It is well known the irregular endocrine islet dysmorphic pancreas in GK rats11. However, we focus in the islets changes after this surgery.
We notice that massive intestinal resection is not a pure malabsorptive surgery, as biliopancreatic diversion or similar procedures could be. Since the biliopancreatic excretions were conserved, the 50% jejunum intestinal resection (IR50) did not reduce nutrient absorption. Instead of this, absorption was conserved with an increased course to reach the ileum.
Materials and methods
Animals
All animal procedures were approved by the Committee for Ethical Use and Care of Experimental Animals at Cádiz University. This committee ensured that the procedures in all experiments were performed in accordance with international relevant guidelines and regulations of animal welfare. The internal Committee for the Ethical Use and Care of Experimental Animals followed the instructions marked for the Andalusian Autonomous Authority.
Twelve males Goto-Kakizaki (GK), diabetic non-obese rats weighing 200-220 g, at an age of 10-11 weeks, were provided and kept at the Experimentation and Animal Production Service of University of Cádiz (SEPA). Female rats were not used to avoid the cyclic variations in gonadotropins and their effect on glycemic metabolism. The male GK rats were stabled in randomized groups (n = 6 each group), under constant temperature and humidity conditions in a 12-h light/dark cycle, with ad libitum access to normal chow and water.
Surgical procedures
The fasting control group (FC) was subjected to the same preoperative and postoperative conditions as the surgical group, with a 12 h-fasting pre- and post-surgical period. The surgical group, the intestinal resection of 50% of jejunum (IR50%) was performed under general anesthetic with continuous infusion of Isofluorane 3% V/V (Isoflo, Abbott 571329.8). The IR50 and FC groups were subjected to a pre- and post-surgical 12-h fasting period. After this period, an intake re-adaptation 24-h period followed each surgery to normalize fasting.
IR50 as the malabsorptive bariatric surgery was performed with the following steps. A laparotomy of about 3 cm in the midline of the abdomen. We identified the angle of Treitz and the ileocecal valve as anatomical references. The bowel between these points was exposed and measured. A resection of the central 50% was made, followed by an end-to-end anastomosis with 5-0 monoplane silk suture (polypropylene, Ethicon Prolene), leaving the proximal half of the jejunum and the distal half of the digestive tube. The ileum was left untouched. Finally, instillation of physiological saline at 37°C in abdominal cavity and closure of the abdominal wall in one layer was done. These final steps were repeated in every surgical procedure.
Sacrifice and tissue preparation
Animals were sacrificed four weeks after surgery by isoflurane inhalation overdose. Pancreas were immediately removed, weighed (precision scale Ohaus Pioneer Mod PA 3102), and were fixed in Bouin’s solution overnight at 4°C. Later, the samples were dehydrated, embedded in paraffin and cut into serial 10 μm microtome sections for immunostaining.
β-cell mass quantification
To calculate β-cell mass, insulin producing cells were stained using a monoclonal mouse anti-insulin antibody (Sigma-Aldrich, I-2018 USA); a secondary biotin conjugated goat anti-mouse IgG antibody and a streptavidin complex (Sigma, Mouse Extra-2). The peroxidase-ABC complex was revealed with solution of 0.3 mg/ml of 3, 3’Diaminobenzidine (Sigma, D5905) in presence of 0.2 μl/ml of H2O2 under microscopic control and counterstained with Harris’s hematoxylin.
The insulin-positive areas were measured using a microscope equipped with a digital camera and the Image J image analysis. Those who performed the measurements were not aware, of which experimental group the samples belonged to. β-Cell mass was measured as an insulin-positive area/total pancreatic area ratio by the total pancreas weight, and it was expressed in mg. Positive, negative, and blocking with BSA samples were carried out as controls to ensure the immunohistochemical techniques.
Histomorphometry
The histomorphometric examination of pancreatic islets was performed in immunostained pancreas sections. Number of β-cell islets/pancreas area μm2 as quantified in a total of 20 islets per condition using a microscope equipped with image analysis Cell D software (Olympus, Hamburg, Germany). Lately, measured islets were distributed and grouped by size. The insulin-positive areas were measured using a microscope equipped with a digital camera and the image analysis Image J. Those who performed the measurements were not aware of which experimental group the samples belonged to.
Statistical analysis
Measurement data were expressed as mean ± SEM. Comparisons between the different groups were performed using the ANOVA test. Once the non-parametric samples character was established, these data were analyzed with Mann–Whitney U-test and p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS statistical software, version 24.0.
Results
β-cell mass
Pancreatic β-cell mass quantification was measured for both groups as described. One month after intestinal resection, β-cell mass was significant increased among the IR50 versus the Sham groups (Fig. 1) (p < 0.05). β-cell mass measured was (18,96 mg in IR50 versus 11,76 mg in Sham group).
Histological analysis of pancreatic islet
Islet histomorphometry study was carried out for both groups of animals, including two parameters: number of endocrine islet per mm2 of pancreatic area and mean area of the Langerhans islet (μm2).
No differences were observed neither the mean size of the islets nor the mean number of these islets in the pancreas of both groups. The number of islets were observed similar (1,46 in IR50 vs. 1,41 Sham islets/μm2) (Fig. 2). No abnormalities were observed in these islets. These were normally distributed across the surface of the pancreas.

Figure 2 Islets histomorphometry: Number of islet in pancreas in each group expressed as islet number/mm2 of β-pancreatic area. No differences appeared in the surgical studied group versus de control group.
About the mean size of the pancreatic endocrine islet, no statistical differences appeared between the groups (6313,79 IR50 vs. 6818,93 Sham islet sizes/μm2) (Fig. 3). Finally, we showed the number of islet per pancreas area in a graph. This distribution spread was according to a size distribution sequence (Fig. 4). The IR50 islet was perceptibly larger than surgical control in all the islets distribution, but not in the little-sized islets (< 100 μm2). This result was not statistically corroborated. The IR50 islets were significant bigger than Sham islets in the 5000-10000 mm2 range.
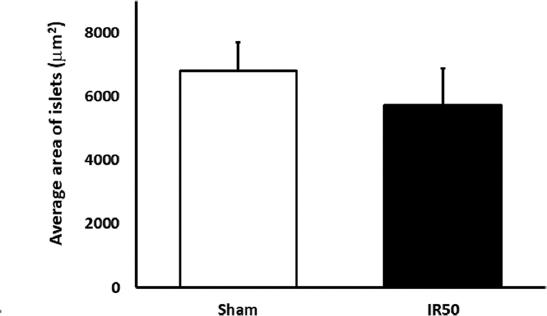
Figure 3 Islets histomorphometry: Mean islet size expressed as mean islet area, as mean ± SEM, in μm2. No statistical significant differences appeared between any studied group.

Figure 4 Islets histomorphometry: Mean islet size distribution quantified in pancreatic area, represented in bar graph as means of islet number/mm2, distributed by grouped sizes. The surgical aggression on the jejunum implied a relative increase in the mean size of islet. The normal mean size of Sham group (around 1500-5000 mm2) contrasted with the augmented size rank (5001-10000mm2 and > 10000 mm2) observed in IR50. The IR50 group showed and important attendance in the rank of medium and great size (form 1500 to > 10000 mm2 islets).
Discussion
Some previous papers have found a late consequence to bariatric surgeries on pancreas cellularity. Thus, pancreatic hormonal release could be in the basis of T2DM improvement after these surgical procedures. Beta-cell cellular changes can be related in the variations of insulin release or peripheral resistance12,13. These arguments have been broadly employed to explain the T2DM improvement. Therefore, we decided to study the changes in beta-cell cellularity in an experimental model. This is not a clinical bariatric procedure. Instead of this, this is an malabsorptive intestinal massive resection model, in which we study the cellularity consequences on beta-cell mass.
Some reports had published changes in endocrine pancreas cellularity, with important differences between restrictive versus malabsorptive techniques9. However, no much consideration had received the analysis of the cellular changes. In this sense, we approach the analysis of the islet as a functional unity. The changes in the islets unit and the number or size of the islets must be the final consequence of functional homeostatic after surgeries.
After performing a severe malabsorptive surgery with 50% of intestinal exclusion (IR50) in GK rats, this technique had a positive effect on β-cell mass, as shown in figure 1. This data is consistent with previous reported results14. The previous experience was performed on healthy Wistar rat model that reported an increased beta-cell mass. Our results confirm the capacity of malabsorptive bariatric surgery to replenish the β-cell population, not only in healthy models but also in pathological environment as T2DM as in GK rats.
A similar beta-cell population enhance after malabsorptive surgery could be the explanation behind plasma glucose levels ameliorate in GK rats after Biliopancreatic diversion (BPD) reported by15.
B-cell increase in our model seems not to be at the expense of an elevate number neither of high or medium size (5001-10000 mm2) islets. Even, small islets do not appear to be increased, as shown in figure 4. This data indicates a growth mechanism based on an increased beta-cell proliferation ratio. Similarly, a high Ki67 positive beta-cell number was found in healthy rat models after IR509. These could imply a protective mechanism over the pancreas after malabsorptive techniques. The beta-cell increase must be related to cellular mechanism, as hypertrophy or hyperplasia. Thus, after a malabsorptive technique the glucose absorption will be faster. Endocrine pancreas could be aggressed by this increased absorption in a long-term adaptation. We suppose the reported increase in beta-cell is a protective solution for endocrine pancreas. The increased beta-cells will release insulin after hyperglycemia, without a maintained stressful on healthy endocrine cells.
High GLP-1 plasma level was found in GK rats after RYGB, a bariatric technique with a remarkable malabsorptive compound. GLP-1 has been broadly implied in the increase and promotion of beta-cell. The possibility of increased GLP-1 plasma level in our model could be consistent, according to previous release results, reported in intestinal mucosae studies16. This GLP-1 increase could act as a trigger for beta-cell proliferation17.
We cannot obviate other options in this cellular adaptation. This could be a trans-differentiation process, where some endocrine lineage cells -as alpha-cells- can be transformed into beta-cells. These mechanisms were described under stress conditions8, such as a high plasma glucose level for a long time, in rodent models18.
However, a trans-differentiation mechanism would imply the overall growth of all islets. In IR50 rats but no one statistical difference around average islet area appears versus Sham rats, as shown in figures 2 and 3. On the other hand, these pancreatic histological events have been analyzed only 4 weeks after surgery. Some phenomena, such as trans-differentiation processes, may take longer to develop.
Taking together, these data lead us to think about a landscape were the short-term response of endocrine pancreas to a severe malabsorptive surgery is a remarkable beta-cell mass expansion. This beta-cell increase is able to replenish β-cell loss due to diabetes development8. This change probably results as an increased β-cell proliferation ratio, as it suggests the size and proportion of islet.
This β-cell increase is report in T2DM; but previously, it was shown in healthy models9. We cannot compare the increase ratio between both models due to the survival period in the experiences. Otherwise, the initial pathophysiological conditions of the islets in GK rats can be related to our results, because β-cells are clearly reduced before surgery. Hence, the potential capacity to increase is reduced in GK rats. Otherwise, the survival period is 1 month in our experience versus 3 months in the previous reports. This can be a reduced time to explore all the cellularity capacity to differentiate.
These data enhance the capability of malabsorptive surgery to trigger short-term histologic endocrine pancreas changes and claim it as a powerful experimental tool to investigate the pathophysiological basis of diabetes and the mechanisms beyond it resolution following bariatric surgery.











 nova página do texto(beta)
nova página do texto(beta)



