Introduction
Chronic wounds currently affect more than 6 million people in the United States, resulting in billions of dollars in health-care costs each year1. In Europe, meanwhile, nearly 2 million people suffer from acute or chronic wounds2. This generates a pressing worldwide health problem causing a substantial burden on global health both economically and socially.
The process by which wounds heal has traditionally been divided into three main stages: inflammation, cell proliferation, and remodeling. Nowadays, however, this is understood to be much more complex than a simple three-stage phenomenon3-5. Eicosanoids, cytokines, growth factors, and nitric oxide are the inflammatory mediators implicated in wound repair, and the cell response involves platelets, neutrophils, macrophages, monocytes, fibroblasts, keratinocytes, endothelial cells, T lymphocytes, and possibly resident stem cells6,7. It is possible that both the signals and the cells above mentioned are altered by different mechanisms associated with some pathologies.
Wounds that fail to heal usually become arrested in the chronic inflammation phase, where both systemic factors and cell migration could be altered, rendering them unable to undergo the normal healing process8. In recent years, many studies have sought to further the understanding of the biology and pathology of wound healing, though much remains to be learned about the treatment of chronic wounds9. To address this issue, emerging cell therapies, including those that employ adipose-derived stem cells (ASCs), have become therapeutic alternatives for tissue regeneration10,11.
Adipose tissue is a source of mesenchymal stem cells, which are widely distributed throughout the body. These cells are capable of secreting several factors that stimulate the wound-healing process, such as TGFα, VEGF, KGF, FGF2, PDGF, HGF, fibronectin, and collagen, among others12; in addition, ASCs also secrete cytokines and chemokines, which can regulate the inflammatory process during healing13-15. Many studies suggest that ASCs can improve tissue regeneration through paracrine mechanisms and have the capacity to stimulate other cells such as keratinocytes and fibroblasts16,17. They exhibit substantial anti-inflammatory and regeneration activity and are easy to obtain, expand, and culture18, and many ongoing clinical trials are using human adipose-derived stem cells to treat damaged tissues in several diseases.
To explore the viability of ASCs to benefit wound healing, the previous studies by our group has analyzed the characteristics of ASCs derived from either obese or cancer patients whose risk of poor healing discouraged use of these cells as therapeutic tools19-22. Obesity, defined as abnormal or excessive accumulation of fat, is associated with low-grade chronic inflammation of adipose tissue19, possibly impairing health20. In cancer patients, tumors activate molecular and cellular mechanisms linked to impaired wound healing21,22. This impairment is also affected by the systemic toxic effect of the chemotherapy drugs and radiation therapies these patients frequently receive23,24. In light of these difficulties, we analyzed ASCs from different patients, studying their phenotype, proliferation, migration activity, differentiation potential, and cytokine secretion to determine the ways in which these cells could influence the healing process25,26. In addition to comparing the differences between the cells of the different pathological groups, at a functional level the migration capacity of the different cell groups, using a protocol described by Grada et al.27 also known as the “in vitro scratch assay;” It was analyzed using in all cases the same plasma enriching the cell culture medium, plasma from a donor without associated pathologies, it could be considered to be equivalent to an allogeneic graft (allogeneic use), since histocompatibility studies were not analyzed between the donor and the patients from whom the cells came.
Materials and methods
Patients samples
The study protocol is adhered to the ethical guidelines of the 1975 Declaration of Helsinki, a prerequisite for the approval granted by the Institutional Ethics Committee of the Fundación Jiménez Díaz University Hospital, Madrid, Spain (PIC number 23/2015_FJD). All patients provided written informed consent.
ASCs were obtained from three different donor groups undergoing elective surgery in the Fundación Jiménez Díaz University Hospital located in Madrid, Spain. These groups included healthy donors (n = 4), obese patients (n = 7), and cancer patients (n = 5). Obese patients were subjects having either a body mass index (BMI) ≥ 35 with associated comorbidities or a BMI ≥ 40 who had undergone elective laparoscopic gastric bypass surgery. Cancer patients were subjects diagnosed with gastric and esophageal cancer. These patients had undergone elective total gastrectomy or esophagectomy (n = 1), transhiatal esophagectomy (n = 1), or three-field minimally invasive esophagectomy (n = 3). A very homogeneous group of patients have been selected in terms of location and tumor stage. Considering that all samples (blood and adipose tissue) are collected before and at the beginning of the surgery, respectively, we can limit the variability between the collected samples. Hematological and biochemical parameters were collected for all (Table 1).
Table 1 Patients’ clinical features
| Healthy | Obese | Cancer | |
|---|---|---|---|
| Age (years) | 43.75 ( ± 7.93) | 39.43 ( ± 6.97) | 56.60 ( ± 10.88) |
| Gender (Male/Female) | 75%/25% | 14.3%/85.7% | 100%/0 |
| BMI (kg/m2) | 26.25 ± 4.03 | 44.14 ± 5.46 | 23.6 ± 4.04 |
| ASA score* | ASA ≤ II: 4 (100%) | ASA ≤ II: 4 (57.1%) ASA > II: 3 (42.9%) |
ASA ≤ II: 4 (80%) ASA > II: 1 (20%) |
| Comorbidities | |||
| Smoking | 25% | 14,3% | 40% |
| Alcohol consumption | 0 | 0 | 20% |
| Hypertension | 0 | 14.3% | 20% |
| Hematological and biochemical parameters | |||
| Hemoglobin (g/dl) | 14.73 ± 1.54 | 14.11 ± 1.12 | 13.10 ± 2.67 |
| Absolute lymphocyte count (×103 ml) | 2.45 ± 0.82 | 2.16 ± 0.99 | 2.14 ± 0.64 |
| Glucose (mg/dl) | 88 ± 6.78 | 95 ± 15.58 | 90 ± 4.42 |
| Urea (mg/dl) | 32 ± 8.29 | 28.20 ± 6.87 | 25.25 ± 7.22 |
| Creatinine (mg/dl) | 0.9 ± 0.08 | 0.74 ± 0.15 | 0.84 ± 0.11 |
| Plasma protein (g/dl) | 7.2 ± 0.14 | 7.17 ± 0.55 | 6.3 ± 0.95 |
| Albumin (g/dl) | 4.35 ± 0.24 | 4.3 ± 0.35 | 3.82 ± 0.31 |
| AST (UI/l)e | 25 ± 8.72 | 20.67 ± 8.17 | 28.4 ± 10.36 |
| ALT (UI/l)c | 24 ± 9.54 | 28.86 ± 14.61 | 37.25 ± 20.42 |
| Total cholesterol (mg/dl) | 180 ± 17.01 | 193.43 ± 21.31 | 178.67 ± 19.09 |
| Triglyceride (mg/dl) | 97 ± 26.51 | 141 ± 78.48 | 119.33 ± 68.66 |
| HDL cholesterolb | 49.50 ± 9.19 | 39.71 ± 10.18 | 60.33 ± 6.51 |
| LDL cholesterolò | 128.5 ± 12.02 | 122.14 ± 19.54 | 97.33 ± 20.43 |
| Cholesterol/HDL | 3.88 ± 0.59 | 5.38 ± 1.55 | 2.98 ± 0.42 |
| Iron (mg/dl) | 93.33 ± 40 | 78.43 ± 18.17 | 88.67 ± 84.3 |
| Transferrin (mg/dl) | 282.33 ± 60.29 | 278.83 ± 45.46 | 251.67 ± 71.57 |
| Glycoside hemoglobin -HbAc1 (%) | 5.25 ± 0.07 | 6.2 ± 1.72 | NA |
*ASA: The American Society of Anaesthesiologists (ASA) Physical Status Classification System is used to establish a person’s functional capacity. ASA grades are a simple scale describing a person’s fitness to be given an anesthetic for a procedure:
ASA I A normal healthy patient. ASA II A patient with mild systemic disease. ASA III A patient with severe systemic disease. ASA IV A patient with severe systemic disease that is a constant threat to life. ASA V A moribund patient who is not expected to survive without the operation. e AST: aspartate aminotransferase c ALT: alanine aminotransferase b HDL Cholesterol: High-density lipoprotein cholesterol. ò LDL Cholesterol: Low-density lipoprotein cholesterol. NA: not applicable.
Clinical, hematological, and biochemical features of different patients used in the study. All values are shown as mean ± standard deviation. The most relevant values appear in bold type.
ASC isolation and cell culture
Cells were isolated and cultured according to the protocol previously published by our laboratory28, though with slight modifications for adipose tissue biopsy. Briefly, adipose tissue samples were thoroughly washed with phosphate buffered saline (PBS) and digested mechanically with scissors and enzymatically using 0.075% collagenase type I (Gibco®, Invitrogen Life Technologies, San Diego, CA, USA) for 1 h at 37°C under constant agitation. These digested samples were then inhibited using fetal bovine serum (FBS) and centrifuged, and the cell pellet was resuspended in Dulbecco’s modified Eagle’s medium high-glucose (DMEM) plus 10% FBS and 1% penicillin/streptomycin. Cells were incubated at 37°C in 5% CO2. Each experiment was performed at least in triplicate, using cells in passages 2-7.
Flow cytometry analysis
The analysis was performed using monoclonal antibodies against CD90, CD73, CD29, CD45, CD34, and HLA-DR (Merck-Millipore, Billerica, MA, USA) according to the minimal criteria established by the International Society of Cell Therapy to verify their status as mesenchymal stem cells29. Briefly, cells in passage 2 were trypsinized, washed, and resuspended with PBS and incubated with the specific antibodies at 4°C for 30 min and analyzed using a BD FACSCanto II flow cytometer (Becton Dickinson, San Jose, CA, USA). At least 10 000 events were obtained in each case.
Cell differentiation
The cells were differentiated following the manufacturer’s instructions (StemPro® Adipogenesis Differentiation Kit and StemPro® Osteogenesis Differentiation Kit, Gibco®). Briefly, we seeded the cells in a 12-well plate at a density of 10 000 cells/cm2 for adipogenic differentiation and 5000 cells/cm2 for osteogenic differentiation, and these cells were incubated for 14 or 28 days, respectively. The specific differentiation medium was replaced every 3-4 days. After specific periods of culture, differentiated cells were detected with Oil Red O staining for adipogenic differentiation and Alizarin Red S staining for osteogenic differentiation. Finally, staining intensity was measured by spectrophotometry: Oil Red O was extracted with 100% isopropanol and measured at 510 nm, and Alizarin Red S was extracted with cetylpyridinium chloride in 10 mM Na2HPO4 (pH7) and measured at 540 nm.
Cell proliferation assay
Cell proliferation was analyzed by AlamarBlue® (AbDSerotec, Oxford, United Kingdom) assay according to the manufacturer’s protocol. Briefly, cells were seeded in a multiwell plate containing 48 wells at a density of 10 000 cells/cm2. After 24 h, AlamarBlue® was added at a concentration of 10% to each well and was incubated for 4 h. Fluorescence was measured in a microplate reader (EnSpire® ultimo de Plate Reader, Perkin Elmer, Waltham MA, USA) at days 1, 4, and 7. The samples were analyzed in triplicate in all cases.
Wound healing scratch assay
Wound healing was assessed by scratch assay performed in triplicate using multiwell culture dishes containing 12 wells each. Cells were seeded at confluence and allowed to attach and form a cell monolayer. After 24 h, a scratch was performed in the cell culture using a 100-μl sterile pin tool to form a cell-free zone to allow cells migration30. Cells were treated with DMEM plus low concentrations of healthy donor plasma at 1% without FBS31, and photographs of the cell cultures were taken at 0, 24, and 48 h. These photographs were analyzed by ImageJ software to measure the wound area (denuded area) at each time point in accordance with Grada et al.27 Denuded areas in control cultures treated with DMEM plus 10% FBS were considered positive controls.
Cytokine analysis
To obtain the cell secretome, cells were seeded in a 48-well plate. At 80% confluence, they were washed 3 times and the medium was replaced with serum-free DMEM. After 48 h, the medium was collected and centrifuged at 1000× g for 10 min. The supernatant was stored at −80°C until use.
To analyze the levels of cytokines present in the secretome released by the cells, the supernatants were tested using MILLIPLEX® MAP Human High Sensitivity T Cell Magnetic Bead Panel (EMD Millipore Corp). The cytokines and chemokines analyzed were ITAC(CXCL11), GM-CSF, Fractalkine, IFNg, IL-10, MIP-3a, IL-12, IL-13, IL-17A, IL-1b, IL-2, IL-21, IL-4, IL-23, IL-5, IL-6, IL-7, IL-8, MIP-1a, MIP-1b, and TNF-α. The experiments were carried out following the manufacturer’s instructions and the levels of cytokines were detected by MAGPIX® technology (Millipore, MA, USA), with all samples analyzed in triplicate.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software. Results are expressed as median values ± SD. Normality was analyzed using the Shapiro–Wilk test and statistical significance was assessed by the Kruskal–Wallis test. Values of p < 0.05 were considered statistically significant.
Results
Patient data
A total of 16 patients were included in the study, and their demographic features are shown in table 1. Obese and cancer patients had a higher rate of associated comorbidities. However, there were no significant differences between the three groups in terms of comorbidities or ASA score.
As concerns hematological and biochemical parameters, the obese patients had higher levels of glucose and glycoside hemoglobin (HbAc1). They also had higher levels of cholesterol, triglycerides, and total cholesterol/HDL ratio, but no statistically significant differences were observed.
The cancer patients showed lower iron and transferrin levels, as well as lower levels of plasma protein. Their levels of albumin were significantly lower (p < 0.05), lending further support to their status as malnourished patients.
Cell morphology
Cells were expanded in tissue-culture flasks and their morphology was examined under a light microscope. Human ASCs derived from different patient groups showed the typical spindle-shaped and fibroblast-like morphology of mesenchymal stem cells (not shown). In all cases remained undifferentiated over time, that is, up to 1 month.
Characterization of ASCs: surface-marker analysis and differentiation potential
A surface-marker analysis of all cells by flow cytometry revealed that ASCs were positive for the expression of CD90, CD73, and CD29, and negative for CD34, CD45, and HLA-DR (Fig. 1A-C). This phenotype remained constant in all groups and passages (between 2 and 7), though obese and cancer patients showed lower proportions of CD29 and CD73 positive cells as compared to healthy donor cells.
Human ASCs from patients were differentiated into adipocytes using commercial differentiation media after 14 days of induction. At that time, the cells were stained with Oil Red O and measured by spectrophotometry at 510 nm.
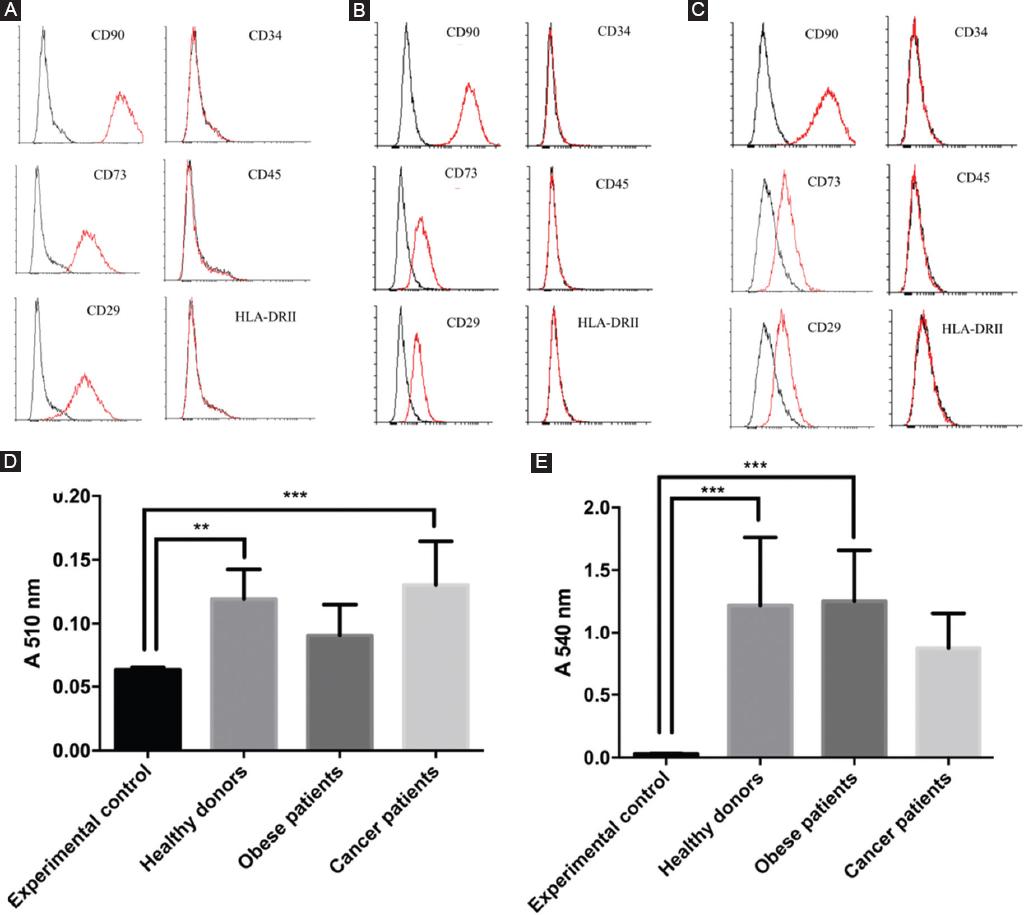
Figure 1 Phenotypical characterization. Characterization of cultured cells by flow cytometry using 3 cell markers positive for MCS (CD90, CD73, and CD29) and 3 cell markers negative for MSC (CD34, CD45, and HLA-DR). A, Cells from healthy donors; B, from obese patients; C, from cancer patients. D, mean level of adipogenic differentiation of ASCs from different patients (absorbance of Oil Red O). E, mean level of osteogenic differentiation of ASC from different donors (absorbance of Alizarin Red S).
All analyzed human ASCs showed adipogenic differentiation by absorbance at 510 nm, but there were no statistically significant differences between samples from healthy donors (0.12 ± 0.02), cancer patients (0.13 ± 0.03), and obese patients. (0.091 ± 0.02), apart from a lower differentiation capacity observed in the cells of obese patients (Fig. 1D). Accordingly, the number of cells with lipid droplet count per field (10 fields/plates were analyzed at random) showed a similar pattern in the different ASCs studied (Supplementary Fig. 1A-D).
The efficiency of osteogenesis was evaluated after 28 days of culture with differentiation culture media after ASC staining with Alizarin Red S and analysis by spectrophotometry at 540 nm. All analyzed human ASCs were positive for osteogenesis and values were significantly higher in cells isolated from both healthy donors (1.22 ± 0.54) and obese patients (1.25 ± 0.40) than cancer patients (0.87 ± 0.28) (Fig. 1E), according to microscopy count (Supplementary Fig. 1E-H).
In vitro migration capacity
We did not observe any significant differences between study groups when analyzing the migration and healing potential of ASCs by means of a scratch wound assay at passage 3-4. The percentage of cell-free wound area at 24 h was 49.5 ± 8.89 for healthy cells, 58.4 ± 8.40 for those derived from obese patients, and 49 ± 18.57 for cancer-patient samples, whereas at 48 h, this percentage was 22.25 ± 14.12, 29 ± 15.12 (p = 0.5267), and 28.52 ± 14.77, respectively (Fig. 2).
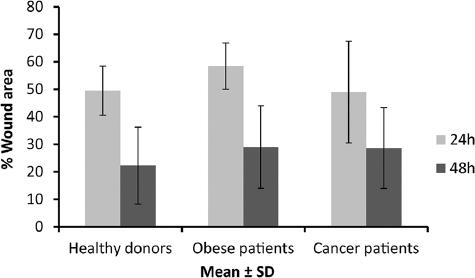
Figure 2 Wound healing scratch assay. Graphic representation of in vitro wound healing scratch assay using plasm of healthy (allogenic grafts) at 24 (grey) and 48 h (white) of ASCs from different donors (healthy, obese, and cancer). In all cases, measures (mean ± SD) of the wound area (denuded area) at each time point are represented.
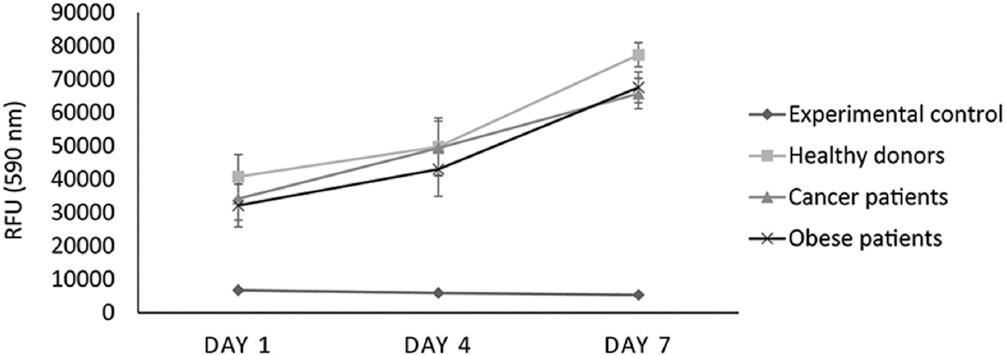
Cell proliferation
We examined cell proliferation for 1 week, measuring this at a fluorescence intensity of 590 nm at days 1, 4, and 7 by alamarBlue® assay. We found that ASCs derived from obese and cancer patients proliferated less than those of healthy controls at day 7, although the differences were not statistically significant at any time point (Fig. 3).
Secretome profiles
To study the secretome of ASCs, we used a magnetic bead panel with which we detected 21 cytokines and chemokines (Table 1, Supplementary Table 1). In table 2, we only show those that showed statistically significant variations, We found low expression of pro-inflammatory (MIP3a, IL8, and TNFα) and regulatory (GM-CSF, Fractalkine, IL6, IL7, and IL21) cytokines and chemokines in ASCs derived from obese patients, observing significant differences (p < 0.01) in all these values as compared to those of ASCs derived from healthy donors (Table 2). In ASCs derived from cancer patients, the values of GM-CSF, MIP-3a, TNF-α, and IL8 showed significant differences (p < 0.01) against those observed in healthy cells (Table 2).
Table 2 Secretome analysis
| Healthy | Obese | Cancer | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p value | Mean | SD | p value | |
| GM-CSF | 53.26 | 10.4 | 3.68 | 0.01 | 0.027 | 183.69 | 92.6 | > 0.99 |
| Fractalkine | 43.74 | NA | 0 | NA | > 0.99 | 45.18 | 13.13 | 0.37 |
| MIP-3a | 9.86 | 1.95 | 3.40 | 1.15 | > 0.99 | 22.97 | 0.25 | 0.20 |
| IL-21 | 4.77 | 0.85 | 0.77 | 0.02 | 0.363 | 4.72 | 0.22 | > 0.99 |
| IL-6 | 1356.40 | 396.27 | 292.15 | 90.10 | 0.024 | 1235.42 | 874.20 | > 0.99 |
| IL-7 | 11.67 | 1.65 | 3.37 | 0.39 | 0.176 | 14.03 | 2.91 | > 0.99 |
| IL-8 | 5990.13 | 28 | 44.75 | 12.09 | 0.522 | 7513.04 | 941 | > 0.99 |
| TNFα | 2.01 | 1. 90 | 0 | NA | 0.004 | 0 | NA | 0.0009 |
Proteins of the secretome revealing differences between cultured cells derived from either healthy individuals or patient groups (obese and cancer). Mean is expressed in pg/mL. ND: not detected, NA: not available. Statistically significant values appear in bold type.
Discussion
Numerous studies have provided evidence of the involvement of ASCs in the wound-healing process owing to the capacity of these cells to secrete a high number of factors that enhance tissue regeneration11,13,14,32,33. Many clinical trials have shown the therapeutic potential of these cells34,35. Overall, the ease with which ASCs may be isolated and expanded in vitro, their abundance in adipose tissue, and their regenerative potential make them an exceptionally good cell type for this kind of therapy. However, several factors may impair their qualities, and little is known about the effect of obesity and cancer on the behavior of ASCs36-38.
In the present study, we have phenotypically and functionally characterized ASCs derived from either obese or cancer patients, comparing these cells to the behavior of healthy ASCs in an attempt to evaluate their potential for therapeutic use for wound repair.
In general, greater similarity is seen between the behavior of ASCs derived from cancer patients and healthy cells than with ASCs isolated from obese patients. However, although the obese patients studied had hyperglycemia as well as higher HbAc1, total cholesterol, triglycerides, LDL, and cholesterol/HDL levels, and lower HDL levels, no statistically significant differences were found when compared to healthy donors. This may be because to enter the bariatric surgery program, obese patients undergo very strict monitoring by an endocrinologist, causing them to lose 5%-10% of their baseline weight and it could has triggered the ASCs and equated their status to the ASCs of the healthy controls. These measures lead to a slight improvement in their metabolic status, partially normalizing their capacity for wound healing39,40. Comparatively, cancer patients had lower BMI and tended to be malnourished, as evidenced by significantly lower levels of albumin, which affected their metabolic status41. Indeed, the sample included patients with esophageal and gastric cancer who often experience difficulties with oral intake.
Attempts to establish correlations between phenotypical changes observed in ASCs derived from patients and their capacity for wound healing are met with challenges42. It is known that CD73 regulates purinergic signaling through the hydrolysis of ATP/ADP to adenosine, modifying the microenvironment42, marking a key factor for wound healing in which cell migration plays an essential role43. Thus, it was relevant to analyze the migration capacity of the ASCs of these patients38. It is also known that CD29 (a β1 integrin chain) plays an important role in mediating the cell-matrix adhesive properties of epithelial cells implicated in wound healing, a topic not analyzed in the current study. However, our results do not demonstrate changes in the capabilities of in vitro wound repair44. Despite this observed trend, our data cannot confirm these differences in the migration capacities of cells from different patients; we consider that a larger sample size could clarify this trend in the future.
ASCs derived from cancer patients and healthy ASCs showed no differences in their morphology, proliferative capacity, and results of wound healing scratch assays. The only differences concerned the expression of two molecules used as MSC markers, that is, CD29 and CD73, and a decreased capacity to differentiate to bone cells, the latter finding would be consistent with the non-prevalence of bone metastasis in patients with esophageal cancer45. Finally a significative higher values in the production of Fractalkine and MIP-3alfa, both chemokines are involved in inflammatory processes of the epithelium, on the one hand, attracting T cells and monocytes and, on the other hand, activating Th17 and therefore attracting B cells and T cells for recoil and inflammatory processes, the increase in both chemokines has already been observed in inflammatory processes associated with tumors46.
On the other hand, ASCs derived from obese patients were similar to healthy ASCs, except for the proportions of cells expressing CD29 or CD73, a lower capacity to differentiate into adipocytes, as previously reported42,47, and significantly lower values in the production of GM-CSF, IL6, and TNFα. Despite these changes, cell migration and repair evaluated in vitro in wound healing scratch assays remain unchanged, thus suggesting that these cells are efficient for wound healing.
Explanations remain elusive as regards the increased production of certain cytokines, including GM-CSF, IL6, and TNFα, in ASCs isolated from obese patients, as adipose tissue is an active organ that secretes a large variety of factors, such as leptin, adiponectin, TNF∝, IL-6, MCP-1, and CCL2, among others48. Moreover, in obese individuals, adipose tissue is associated with low-grade chronic inflammation and is characterized by macrophage infiltration, a source of the pro-inflammatory factors that enhance the secretory activity of adipocytes49. This low-grade of chronic inflammation in obese samples could be a consequence of the treatment patients undergo prior to the collecting of samples (i.e., diet and monitoring of physical activity) or the effect of the culture medium. However, we cannot rule out the possibility that the cells of patients with cancer or obesity actually have different secretory profiles, although their functional significance resists conclusive understanding. We consider that there are two factors that must be analyzed before reaching any conclusion: The microenvironment in which the cells are found in the body, and the signals that the different pathologies “throw” into the bloodstream.
Conclusions
We demonstrate that ASC differentiation potential and cytokine secretion are slightly modified when cells are derived from either obesity or cancer patients, but their proliferation and in vitro wound-healing capabilities are unchanged. Taken together, these findings suggest that, although the ASCs could themselves be involved in the decreased healing capacity seen in these pathologies, the influence of the microenvironment and the signals received through the plasma could be the main cause of the reported defects in healing. New studies to confirm these aspects are necessaries. In addition, this altered behavior of ASCs derived from obese patients or cancer patients might be considered when these cells are used as therapeutic agents. Nevertheless, larger-scale studies and in vivo studies are needed to reach a definitive conclusion as to the real influence of cancer and obesity on this treatment approach.











 nova página do texto(beta)
nova página do texto(beta)