Introduction
At present, complete axillary lymph node dissection (ALND) is not lacking in comorbidity, so there has been a tendency of decreasing rates of ALND1. Sentinel lymph node (SLN) biopsy has become the procedure of choice to evaluate axillary involvement in patients with breast cancer. In fact, it has replaced ALND as the current standard for axillary staging in patients with breast cancer2. The incorporation of intraoperative SLN biopsy has led to a decrease in ALND, thus reducing the associated comorbidities3. To study the state of the SLN, we use at our disposal a semiquantitative method called one-step nucleic acid amplification (OSNA)4,5, based on the measurement of the amount of cytokeratin (CK)-19 (expressed in more than 95% of breast cancers)6. The OSNA technique has the same value as conventional histological techniques, presenting important benefits for patients5 with clinically negative lymph nodes.
The number of copies of CK19 detected by the OSNA technique indicates the volume of the total tumor load (TTL). Therefore, when the SLN biopsy is negative, ALND can be avoided. In cases with low TTL, the performance of ALND could be safely omitted7,8. In fact, patients with micrometastases in the SLN who are treated with SLN biopsy have a disease-free and overall survival comparable to those treated with ALND9. This has also been observed in T1-2 tumors treated with conservative surgery, radiotherapy, or adjuvant systemic therapy who have ≤ 2 SLNs with macrometastasis7. The number of copies of CK19 mRNA is related to the rate of macrometastasis to nonsentinel lymph nodes (NSLNs) and is a major predictor of metastasis to NSLNs10, but it is not the only predictor of metastasis to NSLNs. Therefore, different authors have tried to identify predictive factors of NSLN metastasis to avoid performing ALND11-14. To establish which patients would benefit from skipping an unnecessary ALND, different cutoff points of mRNA copy number have been determined15-17. We believe that there are clinicopathological features that could help improve the prediction of NSLN metastasis. Therefore, the objective of our work is to determine whether any clinicopathological characteristics can improve the prediction of metastasis over only the mRNA copy number in SLN biopsies.
Material and methods
This was a retrospective observational study that included a total of 824 consecutive patients with breast cancer who underwent SLN biopsy and OSNA between October 1, 2010, and March 31, 2018. The study (registration number: 1004-N-18) was approved January 29, 2019, by Andalucia’s Board of Biomedicine Ethics Committee.
Patients undergoing surgery for invasive mammary carcinoma staged T1-3, expressing CK19, with a clinically negative axilla, and with preoperative axillary ultrasound without positive findings or with lymph node biopsy without evidence of metastasis were included. Patients with neoadjuvant chemotherapy, the previous ipsilateral axillary surgery, recurrence, or extensive ductal carcinoma in situ were excluded from the study.
The following demographic parameters were studied: age, menopausal status, age of menopause, parity, and number of births. The clinicopathological parameters studied were axillary lymphadenectomy, tumor size, histological type (ductal, lobular, and others), multicentricity, multifocality, lymphovascular invasion, and tumor histological grade according to the modified Bloom-Richardson system (tubules, nuclei, and mitosis). In addition, the presence of estrogen receptors (ERs), progesterone receptors (PgRs), and Her-2 protein; Ki-67 status; SLN macro- and micrometastases; NSLN macro- and micrometastases; and the TTL of the SLNs were analyzed.
The immunohistochemical (IHC) classification was based on previously established criteria18: “Luminal A-like:” all of ER and PgR positive, HER2 negative, and Ki-67 low; “Luminal B-like HER2 negative:” ER positive, HER2 negative, and at least one of Ki-67 high and PgR negative or low; “Luminal B-like HER2 positive:” ER positive, HER2 overexpressed or amplified, any Ki-67, and any PgR; “HER2 positive nonluminal:” HER2 overexpressed or amplified, ER and PgR absent; and “Triple-negative ductal:” ER and PgR absent, HER2 negative.
Pre-operative evaluation of the axilla was performed by axillary ultrasound after the diagnosis of breast cancer. Lymph nodes suspected of metastasis were those with a cortical thickening of 2-3 mm, focal bulging, rounded shape, partial or complete loss of fatty hilum, nonhilar blood flow, or partial or complete replacement of the lymph node by tumor tissue. Those suspicious lymph nodes were subjected to a thick-needle biopsy and were discarded in case of metastatic confirmation.
The identification of the SLN biopsy was performed in those patients with clinically and ultrasound-normal axilla using the established protocol with radiopharmaceuticals and blue dye. A 99mTc-labeled albumin nanocolloid was injected periareolarly intradermally (40 MBq the day before surgery). Two milliliters of undiluted methylene blue dye was injected subdermally into the periareolar area of the four breast quadrants (0.5 ml in each quadrant) under anesthesia. Once the SLN was removed, it was sent fresh to the Pathology Department, and OSNA was run. The OSNA technique was performed as described by Tsujimoto et al.4 The amplification rate was measured by spectrophotometry, and the number of copies of CK19 mRNA was calculated compared to a standard curve. The number of copies of CK19 mRNA/ml was determined according to Tsujimoto et al.4: macrometastasis: > 5,000 copies/ml of CK19 mRNA, micrometastasis: 250-5,000 copies/ml; and no metastasis: < 250 copies/ml.
Level II ALND was performed, and NSLNs were studied after being processed by hematoxylin and eosin staining. The tissue blocks of the NSLN were sectioned with a thickness of 3 microns with an interval of 200 microns.
Statistical analysis
Descriptive statistics were performed using the mean and standard deviation for the numerical variables and percentages for the qualitative variables, both in the total sample and in groups defined by the variable ALND with or without metastatic NSLN. The numerical variables between the two groups of ALND with or without metastatic NSLN were compared by Student’s t-test if the normality of the data was verified (Shapiro-Wilk test) or by the Mann–Whitney test if they were not normal. The qualitative variables were compared between the groups by the Chi-squared test or Fisher’s exact test. p < 0.05 was considered statistically significant.
Binary logistic regression models were performed between ALND (with a metastatic/without a metastatic) and each of the variables identified as prognostic of the presence or absence of metastasis in ALND. These models led us to the selection of the variable LogTTL in model 1. The incorporation of variables in the successive models was carried out taking into account the increase in the predictive capacity of the models, their calibration (Hosmer-Lemeshow) and the discrimination capacity of the model (Harrell’s C statistic). The final model was selected for its maximum discriminatory capacity, good calibration, along with its parsimony and interpretability.
The probabilities obtained from the binary logistic regression model were used as a diagnostic test of ALND with metastatic NSLNs, and receiver operating characteristics (ROC) curve analysis was performed for the accuracy of the predictions of the model through sensitivity and specificity. Positive (PPV) and negative predictive values (NPV) were also determined from a cutoff point of the probability. The ROC curves of our model and log (CK19) were drawn, and the areas under the ROC curves (AUCs) of the two tests were compared.
The statistical analysis was performed using the IBM SPSS Statistics program version 24 (IBM, Armonk, NY, USA).
Results
A total of 824 patients with breast cancer were recruited, of whom 118 required ALND. Of these, 42 (35.6%) presented an ALND with a metastatic NSLN, and 76 (64.4%) had ALND without a metastatic NSLN. The general characteristics of the global population (n = 824) and the study population (n = 118) are found in table 1. No significant differences were found in the general characteristics between patients with ALND with a metastatic NSLN and patients with ALND without a metastatic NSLN.
Table 1 General characteristics of the patients studied
| Mean ± SD o % | p | ||||
|---|---|---|---|---|---|
| Global population | Study population | ALND with a metastatic | ALND without a metastatic | ||
| Age | 57.19 ± 12.35 | 55.98 ± 12.92 | 53.90 ± 10.62 | 57.13 ± 13.96 | 0.296 |
| Age groups | |||||
| < 50 | 233/824 (28.3%) | 42/118 (35.6%) | 15/42 (35.7%) | 27/76 (35.5%) | 0.419 |
| 50-69 | 443/824 (54%) | 55/118 (46.6%) | 22/42 (52.4%) | 33/76 (43.4%) | |
| ≥ 70 | 146/824 (17.7%) | 21/118 (17.8%) | 5/42 (11.9%) | 16/76 (21.1%) | |
| Menopausal status | 523/824 (63.5%) | 65/118 (55.1%) | 24/42 (57.1%) | 41/76 (53.9%) | 0.847 |
| Age of menopause | 49.18 ± 4.98 | 48.63 ± 4.86 | 49.46 ± 4.44 | 48.15 ± 5.07 | 0.188 |
| Parity | 722/824 (87.6%) | 105/118 (89%) | 38/42 (90.5%) | 67/76 (88.2%) | 0.769 |
| Number of births | 2.50 ± 1.22 | 2.48 ± 1.36 | 2.50 ± 1.22 | 2.46 ± 1.44 | 0.675 |
ALND: axillary lymph node dissection. The comparison of numerical variables between the defined groups was carried out using the Student’s t-test for independent samples or the Mann–Whitney test, if the data did not meet the normality hypothesis (Shapiro–Wilk test). The association between qualitative variables was made using the Chi-square test or Fisher’s exact test.
Clinical and anatomopathological characteristics of the tumor according to the presence of metastases in the NSLN are shown in table 2. There were no statistically significant differences between the two groups. However, multicentricity was more frequent in ALND with metastatic NSLN (23.8% vs. 10.5%; p = 0.065). The immunohistochemical characteristics of the tumors of the patients studied are shown in table 3. Luminal A tumors were more frequent in patients with ALND with a metastatic NSLN (57.1% vs. 46.1%; p = 0.336).
Table 2 Clinical and anatomopathological characteristics of the tumor according to the presence of metastasis in a NSLN
| Mean ± SD o % | p | ||
|---|---|---|---|
| ALND with a metastatic | ALND without a metastatic | ||
| Tumor size | 53.90 ± 10.62 | 57.13 ± 13.96 | 0.296 |
| Tumor size > 2 cm | 21/42 (50%) | 36/76 (47.4%) | 0.850 |
| Histological type | |||
| Ductal | 30/42 (71.4%) | 61/76 (80.3%) | 0.161 |
| Lobular | 11/42 (26.2%) | 10/76 (13.2%) | |
| Others | 1/42 (2.4%) | 5/76 (6.5%) | |
| Multicentricity | 10/42 (23.8%) | 8/76 (10.5%) | 0.065 |
| Multifocality | 4/42 (9.5%) | 8/76 (10.5%) | 1.000 |
| Lymphovascular invasion | 13/42 (30.9%) | 26/76 (34.2%) | 0.839 |
| Tumor histological grade | 0.438 | ||
| 1 | 8/42 (19.0%) | 8/76 (10.5%) | |
| 2 | 20/42 (47.6%) | 41/76 (53.9%) | |
| 3 | 14/42 (33.3%) | 26/76 (34.2%) | |
| Tumor histological grade (Grouped) | |||
| Less (1-2) | 28/42 (66.7%) | 49/76 (64.4%) | 1.000 |
| Higher (3) | 14/42 (33.3%) | 26/76 (34.2%) | |
| Tubules | |||
| 1 | 3/42 (7.1%) | 2/76 (2.6%) | 0.435 |
| 2 | 10/42 (23.8%) | 23/76 (30.3%) | |
| 3 | 29/42 (69.0%) | 50/76 (65.8%) | |
| Tubules (Grouped) | |||
| Less (1-2) | 13/42 (30.9%) | 25/76 (32.9%) | 0.839 |
| Higher (3) | 29/42 (69.0%) | 50/76 (65.8%) | |
| Nuclei | |||
| 1 | 2/42 (4.8%) | 1/76 (1.3%) | 0.499 |
| 2 | 18/42 (42.8%) | 28/76 (36.8%) | |
| 3 | 22/42 (52.4%) | 46/76 (60.5%) | |
| Nuclei (Grouped) | |||
| Less (1-2) | 20/42 (47.6%) | 29/76 (38.1%) | 0.435 |
| Higher (3) | 22/42 (52.4%) | 46/76 (60.5%) | |
| Mitosis | |||
| 1 | 23/42 (54.8%) | 36/76 (47.4%) | 0.286 |
| 2 | 14/42 (33.3%) | 21/76 (27.6%) | |
| 3 | 5/42 (11.9%) | 18/76 (23.7%) | |
| Mitosis (Grouped) | |||
| Less (1-2) | 37/42 (88.1%) | 57/76 (75%) | 0.148 |
| Higher (3) | 5/42 (11.9%) | 18/76 (23.7%) | |
ALND: axillary lymph node dissection, NSLN: nonsentinel lymph nodes. The comparison of numerical variables between the defined groups was carried out using the Student’s t-test for independent samples or the Mann-Whitney test, if the data did not meet the normality hypothesis (Shapiro–Wilk test). The association between qualitative variables was made using the Chi-square test or Fisher’s exact test.
Table 3 Immunohistochemical profile of the tumor according to the presence of metastasis in a NSLN
| Mean ± SD o % | |||
|---|---|---|---|
| ALND with a metastatic | ALND without a metastatic | p | |
| IHC | |||
| Luminal A-like | 24/42 (57.1%) | 35/76 (46.1%) | 0.810 |
| Luminal B-like HER2 negative | 15/42 (35.7%) | 30/76 (39.5%) | |
| Luminal B-like HER2 positive | 2/42 (4.8%) | 6/76 (7.9%) | |
| HER2 positive nonluminal | 0/42 (0%) | 1/76 (1.3%) | |
| Triple-negative | 1/42 (2.4%) | 3/76 (3.9%) | |
| IHC (Grouped) | |||
| Luminal A-like | 24/42 (57.1%) | 35/76 (46.1%) | 0.336 |
| No Luminal A-like | 18/42 (42.9%) | 40/76 (52.6%) | |
| ER | 41/42 (97.6%) | 72/76 (94.7%) | 0.654 |
| PgR | 33/42 (78.6%) | 66/76 (86.8%) | 0.298 |
| Her2 positive | 2/42 (4.8%) | 7/76 (9.2%) | 0.486 |
| Ki 67 (%) (Grouped) | |||
| ≤ 20 | 29/42 (69.0%) | 43/76 (56.6%) | 0.239 |
| > 20 | 13/42 (31.0%) | 32/76 (42.1%) | |
ALND: axillary lymph node dissection, IHC: immunohistochemical, ER: estrogen receptors, PgR progesterone receptors. The association between qualitative variables was made using the Chi-square test or Fisher’s exact test.
When we compared the characteristics of axillary surgery between patients with ALND with a metastatic NSLN and ALND without a metastatic NSLN, we observed that patients with ALND with a metastatic NSLN had more positive SLNs (1.55 ± 0.67 vs. 1.38 ± 0.65; p = 0.116).), greater rate of macrometastasis (92.9% vs. 67.1%; p = 0.001), and a higher TTL (827070 ± 2585266 vs. 353221 ± 912077; p = 0.005) (Table 4).
Table 4 Sentinel node status according to the presence of metastasis in a NSLN
| Mean ± SD o % | |||
|---|---|---|---|
| ALND with a metastatic | ALND without a metastatic | p | |
| Numbers of isolated SLNs | 1.76 ± 0.79 | 1.78 ± 0.92 | 0.834 |
| Numbers of isolated SLNs > 1 | 24/42 (57.1%) | 39/76 (51.3%) | 0.569 |
| Positive SLNs | 1.55 ± 0,67 | 1,38 ± 0,65 | 0.116 |
| Positive SLNs > 1 | 19/42 (45.2%) | 23/76 (30.3%) | 0.113 |
| SLN | |||
| Micrometastasis | 3/42 (7.1%) | 25/76 (32.9%) | 0.001 |
| Macrometastasis | 39/42 (92.9%) | 51/76 (67.1%) | |
| TTL | 827070 ± 2585266 | 353221 ± 912077 | 0.005 |
| Log TTL | 5.05 ± 0.98 | 4.38 ± 1.21 | 0.005 |
ALND: axillary lymph node dissection, NSLN: nonsentinel lymph nodes, SLN: sentinel lymph node, TTL: total tumor load. The comparison of numerical variables between the defined groups was carried out using the Student’s t-test for independent samples or the Mann–Whitney test, if the data did not meet the normality hypothesis (Shapiro–Wilk test). The association between qualitative variables was made using the Chi-square test or Fisher’s exact test.
The different logistic regression models are reflected in table 5. Multivariate logistic regression showed that log TTL was the factor that best predicted metastasis to a NSLN. The analysis of the ROC curve showed that log (TTL) had an AUC of 0.651 (95% confidence interval [CI]: 0.552-0.751) (Fig. 1, red line). To set the cutoff point of total CK19 mRNA copy number (i.e., the TTL), the one with the greatest sensitivity and specificity was used, captured in the form of the Youden index. This CK19 mRNA copy number was 7294. This cutoff point had a sensitivity of 93%, a specificity of 37%, a PPV of 44%, and a NPV of 91%.
Table 5 Logistic regression models
| Models | Variables | Coefficients | p | OR (IC95%) | Calibration (Homer-Lemeshow) p | Discrimination capacity of the model (Harrell’s C statistic) IC95% |
|---|---|---|---|---|---|---|
| 1 | Constant | −3.081 | 0.001 | 0.878 | 0.65 (0.56; 0.75) | |
| Log TTL | 0.527 | 0.004 | 1.70 (1.18; 2.43) | |||
| 2 | Constant | −2.897 | 0.002 | 0.388 | 0.68 (0.58; 0.77) | |
| Log TTL | 0.549 | 0.003 | 1.73 (1.20; 2.50) | |||
| IHC | −0.581 | 0.156 | 0.56 (0.25; 1.25) | |||
| 3 | Constant | −2.142 | 0.038 | 0.715 | 0.71 (0.61; 0.81) | |
| Log TTL | 0.542 | 0.004 | 1.72 (1.18; 2.50) | |||
| IHC Multicentricity |
−0.555 −0.878 |
0.180 0.106 |
0.57 (0.26; 1.30) 0.42 (0.14; 1.20) |
|||
| 4 | Constant | −2.508 | 0.022 | 0.659 | 0.75 (0.66; 0.84) | |
| Log TTL | 0.660 | 0.001 | 1.93 (1.30; 2.90) | |||
| IHC | −1.186 | 0.018 | 0.31 (0.11; 0.82) | |||
| Multicentricity PgR | −1.104 1.824 |
0.058 0.008 |
0.33 (0.11; 1.04) 6.20 (1.62; 23.64) |
TTL: total tumor load. IHC: immunohistochemical. PgR progesterone receptors.
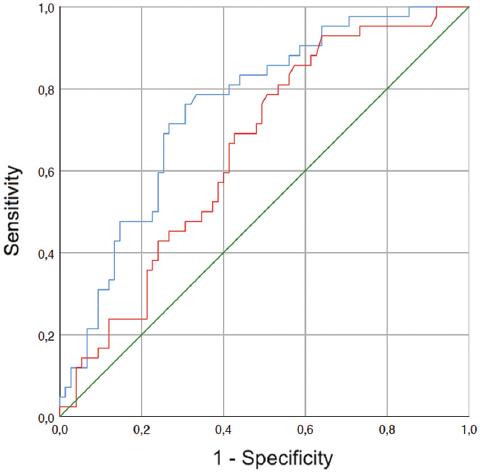
Figure 1 The ROC curves. Red line shows the AUC of the TTL log: 0.651 (IC: 0.552-0.751) (p = 0.007). Blue line indicates the AUC of the TTL log, multicenter, IHC (pooled), and progesterone receptor: 0.752 (CI: 0.663-0.841) (p < 0.0005). ROC: receiver operating characteristic, AUC: area under the curve, TTL: total tumor load, IHC: immunohistochemical.
We correlated the different clinicopathological parameters that were relevant to the prediction of NSLN metastasis with the ROC curve of log (TTL) (Tables 2 and 3). The best AUC was obtained by relating multicentricity and IHC (pooled) to log (TTL). The AUC was 0.752 (95% CI: 0.663-0.552) (Fig. 1 blue line) when incorporating log (TTL) (OR: 1.93; 95% CI: 1.30-2.90), multicentricity (OR: 0.33; 95% CI: 0.11-1.04).
Discussion
Recently, different nomograms based on the TTL of the SLN have been published. The AUC for TTL has varied between studies. Lower AUCs have been described by Teramoto et al. (0.66)19 and Rubio et al. (0.68)20. Other authors, such as Shimazu et al. (0.70)21, Filloppo et al. (0.71)22, Terretano et al. (0.765)17, and Sanguanraksa et al. (0.789)23, present similar figures to ours. Still others have described higher AUCs, such as Nabais et al. (0.805)24 and Fung et al. (0.86)25. We note that we obtained AUCs equivalent to those previously described when we only analyzed TTL19,20. However, we improved the AUC to 0.711 when we added the multicentricity and IHC (pooled) together with the TTL, and this AUC is similar to others previously described17,21-23. Therefore, we believe that clinicopathological factors are of some importance at the time of nomogram design and should be considered even if no statistically significant differences are found among the study population.
Heilmann et al.26 defined a CK19 mRNA copy number cutoff point similar to ours, with a sensitivity (91%) and specificity (61%) similar to what we have described26. However, unlike our work, Heilmann et al.26 combined histological and OSNA techniques for SLN processing, so the CK19 mRNA copy number could be affected. When we observed the reported cutoff points of the TTL of the SLN to predict NSLN metastasis, different authors have reported progressively lower ones15-17. Peg et al.16 in 2013 determined a higher TTL cutoff point, 15,000 copies of CK19 mRNA (sensitivity 76.7%, specificity 55%, PPV 41.1%, and NPV 85.5%). Subsequently, Ambrogio et al.15 defined this cutoff point at 7700 copies of CK19 mRNA (sensitivity 78%, specificity 57%, PPV 50%, and NPV 83%), which is a value similar to that obtained in our study (7294). Finally, in 2017, Terretano et al.17 presented the lowest cut-off point, 2150 copies (sensitivity 94.9%, specificity 51.4%, PPV 46.5%, and NPV 95.8%). Each also reports the sensitivity, specificity, NPV, and PPV of its cutoff point. In our case, with 7294 copies of CK19 mRNA, we defined a sensitivity of 93%, a specificity of 63%, a PPV of 44%, and a NPV of 91%. However, when we added the clinicopathological parameters to the model, we observed a better AUC. This opens an important avenue of research since it establishes the possibility of building statistical models that include clinicopathological parameters to predict metastases in NSLN. The parameters obtained from our ROC curve of TTL are similar to those described by the different authors with different cut-off points15-17. This indicates that no established cut-off point is superior to another, and we should use other parameters to predict NSLN metastasis, which we believe may be certain clinicopathological characteristics.
The influence of clinicopathological characteristics on predicting the risk of metastasis to NSLNs has been described by other authors. Meretoja et al.27 described that HER-2 status and histological and nuclear grade could influence the appearance of NSLN metastasis. Others have described that tumor size is important in NSLN metastasis28-30. In fact, clinicopathological factors have been included in nomograms. For example, Rubio et al.20 included TTL, tumor size, the presence of lymphovascular invasion, HER-2 status, and the number of metastatic SLNs. However, Shimazu et al.21 postulated that nomograms that use various pathological parameters are not practical for intraoperative decisions. We believe that the most important clinicopathological factors can improve the predictive capacity of nomograms and therefore should be considered.
The strongest point of our work is that we have established the basis for determining possible future models that consider clinicopathological factors associated with TTL to improve the predictive capacity for NSLN metastasis. In addition, we have included the fewest number of clinicopathological factors possible to obtain the best AUC, with which we can build future mathematical models that are easy to apply in the clinic. This study does have some limitations, such as its retrospective design, similar to the previous studies, and the small number of patients included. These aspects would be interesting to improve on in future studies.











 nova página do texto(beta)
nova página do texto(beta)


