Introduction
Ischemic enteritis is a vascular disease which presents with a very poor prognosis1. The causes of acute mesenteric ischemia include acute occlusion of the arterial or venous system. Particularly, acute arterial embolization (50%), acute arterial thrombosis (25%), and thrombosis of the mesenteric vein (5%). The non-occlusive intestinal ischemia (non-occlusive mesenteric ischemia) is the result of a decrease in blood supply to the superior mesenteric artery due to visceral vasospasm2-4. The clinical consequences of the disease include ischemic bowel necrosis, sepsis, and death and are related to the time of diagnosis. Therefore, early diagnosis and therapeutic intervention are considered mandatory5. During the last decades, the incidence of ischemic enteritis increases, while the mortality rate remains consistently high (70-90%)6,7. The disease affects patients of different ages and is associated with various conditions such as atherosclerosis, systemic lupus erythematosus, or diabetes mellitus8,9.
Acute mesenteric ischemia is followed by a sequence of events that exacerbate the clinical manifestations of the disease and lead to systemic inflammatory response (SIRS) and multiple organ failure (MODS). This sequence of events includes activation of cytokines, reactive oxygen species, neutrophils and platelet activating factor, by processes such as bacterial overgrowth, disruption of intestinal barrier, bacterial translocation, and bacteremia10-12. Recently, the inflammatory and immunological processes that are triggered by intestinal ischemia, as well as those nutritional agents that could possibly modify patient's outcome are extensively studied, leading to the concept of immunonutrition. This includes, among others, special diets rich in n-3 polyunsaturated fatty acids (PUFA) which are thought to affect the inflammatory and immunological response. Main sources of n-3 fatty acids are oily fish13. Recent studies suggest that metabolites of n-3 fatty acids reduce inflammatory response in compare to arachidonic acid derivatives that arise from n-6 fatty acids14-17.
The present study compares the effects on the inflammatory process of high-molecular solution enriched with n-3 fatty acids, compared to a corresponding artificial high-molecular polymer total enteral nutrition in Wistar rats with controlled narrowing of the superior mesenteric artery leading to ischemic enteritis.
Materials and Methods
Laboratory animals
The animal protocol was designed to minimize pain or discomfort to the animals. For the purposes of this study, we used 40 Wistar rats. The rats were all of the same sex, female, to minimize variations, 2-month-old and weighted between 150 and 200 g. The rats originated from a breeding colony established at the Experimental Laboratory of “Theageneio” Hospital of Thessaloniki where they remained until the age of 2 months. Then, they were transferred at the Experimental Laboratory of the Veterinary School of the Aristotle University of Thessaloniki where they stayed for 7-10 days before the onset of the experimental study. The laboratory environment had a constant temperature, humidity, and ventilation, while special timers secured a 12-h light and dark cycle. The animals were kept in cages in pairs under excellent sanitary conditions and had free access to food and water. The experiments followed the existing provisions of the European Convention for the protection of animals used for experimental and research purposes (N.1197/81 Arthr.4, N. 2015/92, PD 160/91). A veterinarian supervised the whole experimental procedure and the research protocol was approved by the Ethical Committee of the Department of Veterinary Services of the Prefecture of Thessaloniki (S.N.: 13/3564/03.26.2010).
Anesthesia
The surgery was performed under general anesthesia. Initially, a volatile anesthetic was used for a few second, which was achieved by placing the animals in a glass cage which contained gauze impregnated with ether. Then, sodium thiopental solution was injected intraperitoneally at a dose of 30mg/kg of body weight. This anesthetic regimen ensured analgesia and control of the mobility and irritability of the rat for about 1 h.
Surgical technique
After the induction of anesthesia, the abdomen of the animal was shaved and sterilized using 10% Povidone-iodine. Then, it was placed on a sterile operating table, the limbs were immobilized and sterile surgical sheets were placed on the abdominal wall. A midline three-cm abdominal incision was made through which the superior mesenteric artery was recognized. A 23G needle (diameter 0.65 mm) was placed on the vessel and tied with 4-0 silk suture18. The needle was then removed achieving in this way a controlled narrowing of the superior mesenteric artery of about 65-70%19. Blood samples were collected before the procedure, which were centrifuged and stored at −80°C. Finally, the abdominal wall was closed in one layer using a 3-0 silk suture and the surgical incision was carefully cleaned. Net operative time ranged between 15 and 20 min. No antibiotics were administered. After the procedure, the rats were placed in pairs back in the cages and were monitored for about 1 h until fully recovered.
Experimental setup
Ischemic enteritis was induced by controlled narrowing of the superior mesenteric artery, and following the rats were divided randomly into two groups: N3PUFA (n = 20) - Following surgery rats were solely fed with a high-molecular polymer artificial total enteral nutrition enriched with n-3 PUFA in a ratio n-6/n-3 = 0.3/1.0, containing eicosapentaenoic acid and docosahexaenoic acid for 15 days (ProSure®, Abbott 240 ml). The detailed ingredients of the diet are shown in table 1. During the autopsy, blood and tissue samples were taken. Control (n = 20) - Control Group: Following surgery rats were solely fed with a high-molecular polymer artificial total enteral nutrition (Ensure Plus®, Abbott 200 ml) containing alpha linolenic acid for 15 days. The detailed ingredients of the diet are shown in table 1. During the autopsy, blood and tissue samples were taken.
Table 1 Nutritional details of the administrated diet regimens
| Nutrition | Prosure | Ensure plus |
|---|---|---|
| Characteristics | ||
| Energy density | 1.3 kcal/ml | 1.5 kcal/ml |
| Energy distribution | ||
| Protein | 20.9% | 15% |
| Carbohydrate | 57.7% | 57% |
| Fat | 18.1% | 28% |
| Fiber | 3.26% | |
| Renal solute load | 517 mOsm/L | 430 mOsm/L |
| Osmolarity | 507 mOsm/L | 509 mOsm/L |
| Osmolality | 753 mOsm/kg H20 | 680 mOsm/kg H20 |
| Energy (per 1 l) | ||
| Energy | 5360/1270 KJ/Kcal | 6320/1510 KJ/Kcal |
| Protein | 66.5 g | 62.5 |
| Fat | 25.6 g | 49.2 g |
| EPA | 4.5 g | - |
| DHA | 2 g | - |
| ALA | - | 2.75 g |
| Saturated Fat | 7.5 g | 5.5 g |
| Carbohydrate | 183.3 g | 202 g |
| Sugars | 29 g | 67 g |
| Dietary Fiber | 20.7 g | |
| FOS | 11 g | 12 g |
| Salt | 3.8 g | 2.3 g |
| Taurine | 200 mg | 150 mg |
| Carnitine | 100 mg | 120 mg |
| Vitamins (per 1 l) | ||
| Vitamin A (acetate) | 2050 mcg RE | 1170 mcg RE |
| Vitamin A (beta-carotene) | 700 mcg RE | 290 mcg RE |
| Vitamin D3 | 17 mcg | 20 mcg |
| Vitamin E | 200 mg a-TE | 21 mg a-TE |
| Vitamin K1 | 100 mcg | 120 mcg |
| Vitamin C | 430 mg | 120 mg |
| Folic Acid | 1690 mcg | 400 mcg |
| Thiamine (Vitamin B+) | 2.5 mg | 2 mg |
| Riboflavin (Vitamin B2) | 2.9 mg | 2.7 mg |
| Vitamin B6 | 3.4 mg | 2.7 mg |
| Vitamin B12 | 5 mcg | 5.5 mcg |
| Niacin | 25 mg NE | 26 mg NE |
| Pantothenic acid | 11 mg | 11 mg |
| Biotin | 50 mcg | 60 mcg |
| Choline | 510 mg | 550 mg |
| Minerals (per 1 l) | ||
| Sodium | 1500 mg | 920 mg |
| Potassium | 2000 mg | 1600 mg |
| Chloride | 1520 mg | 1100 mg |
| Calcium | 1480 mg | 1200 mg |
| Phosphorus | 1050 mg | 1000 mg |
| Magnesium | 420 mg | 300 mg |
| Ferrum | 6.5 mg | 21 mg |
| Zinc | 250 mg | 160 mg |
| Manganese | 4.2 mg | 5 mg |
| Copper | 780 mcg | 2200 mcg |
| Iodine | 160 mcg | 220 mcg |
| Selenium | 79 mcg | 83 mcg |
| Chromium | 100 mcg | 135 mcg |
| Molybdenum | 140 mcg | 160 mcg |
Pilot studies showed that the daily consumption of the nutrition formula was 20 ml/100 g body weight/day. Hence, the estimated levels of total n3 fatty acids in the N3PUFA group administrated were 0.13 g/100 g body weight/day, so each animal received approximately 0.195-0.26 g of n3 fatty acids per day and in the CONTROL group was 0.055 g/100 g body weight/day, so each animal received approximately 0.0825-0.11 g of n3 fatty acids/day.
Autopsy
The sacrifice was scheduled on the 15th post-operative day. After anesthetizing the animals with ether in a glass cage, the abdominal wall was reopened and blood samples (0.7 ml) as well as tissue samples from the small intestine were received. The blood samples were centrifuged immediately and stored at −80°C to perform the necessary measurements. The tissue samples included resection of a 1-cm part of the small intestine at around 5 cm away from the ileocecal valve which was placed in a 4% formalin solution and sent for histological examination. Finally, the animals were sacrificed by intracardiac administration of KCl 10%.
Body weight
The body weight of every animal was measured at the start of the experiment using a precision scale and, then, again before the sacrifice and the results were recorded based on the code of animal. The average body weight was calculated per group and the changes between the beginning of the experiment and before sacrifice were compared.
Cytokine measurement: interleukin (IL) 1b, IL-6, and tumor necrosis factor a (TNF-a)
The measurement of cytokines IL-1b, IL-6, and TNF-a was made in the blood serum. The samples were collected on the 1st day, before the narrowing of the superior mesenteric artery, and on the 15th day, before the sacrifice. The samples were centrifuged immediately and stored at −80°C. Measurement of cytokines was performed with commercial available ELISA kits.
Histology
Small parts of the terminal ileum were sent for histological examination. After dehydration in alcohol, representative sections were embedded in paraffin and then, from these, 5-m-thick sections were taken and stained with hematoxylin-eosin. The evaluation of ischemic lesions of the intestinal mucosa was made in accordance with the scale of Chiu et al.20, as shown below: 0 = Normal mucosal villi. 1 = Growth of a submucosal space of Gruenhagen's (A space created by the separation of the mucosa from the basal membrane as a result of ischemia). The epithelial cells have a normal appearance at the top of the intestinal villi, often with capillary congestion. 2 = Extension of the submucosal space with moderate elevation of the epithelial layer from the basal membrane. 3 = Marked elevation of the mucosa along the villi and small number of denuded villi. 4 = Denuded villi with exposure of the basal membrane and dilated capillaries. 5 = Digestion, disintegration of the basal membrane, bleeding, and ulceration.
Statistical analysis
After testing for normality, the Mann–Whitney statistical test was employed for the comparison, between the two groups N3PUFA and CONTROL, of body weight (before the experiment and before autopsy), of body weight loss, of cytokines IL-1b, IL-6, TNF-a, and the extend of ischemic lesions. In all of the non-parametric tests, the level of significance (p-value) was calculated by the method of simulations of Monte-Carlo (10,000 resampling)21. With this method safe inferences were achieved, even when the methodological requirements of the statistical tests were not met. The Wilcoxon statistical test was performed to evaluate variations in body weight and blood cytokines between the beginning of the experiment and the sacrifice, for each group. In all statistical tests, the level of significance was predetermined at p ≤ 0.05. Statistical analysis was performed using the SPSS V.15.0 software (SPSS Inc., IL: Chicago, USA).
Results
Body weight
No statistically significant difference was observed in body weight between the two groups at the beginning of the experiment (p = 0.457) as well as at the day of sacrifice (p = 0.091). However, the body weight loss was significantly lower in the N3PUFA group compared to control (p = 0.004). According to the Wilcoxon test, there was a statistically significant decrease in body weight in both groups between the beginning of the experiment and the time before the sacrifice (p < 0.001). All the changes in body weights of both groups are presented as a bar graph in figure 1A and body weight loss in figure 1B.
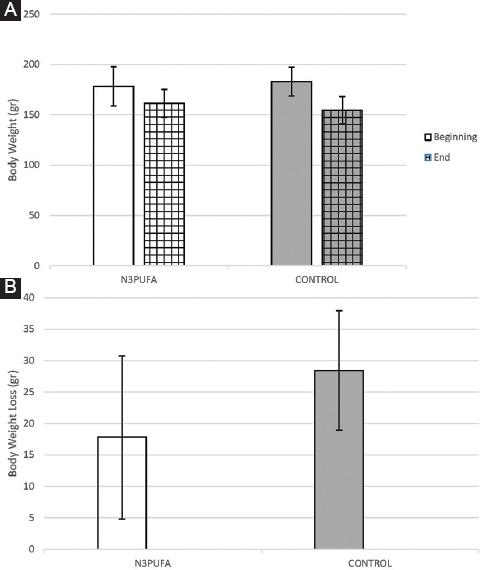
Figure 1 A: comparison of body weight of the two groups at the beginning of the experiment and prior to sacrifice (mean ± standard deviation). No statistically significant difference was noted between the subgroups neither at the beginning nor at the end of the experiment, but the decrease of body weight within each subgroup was statistically significant (p < 0.001). B: comparison of body weight loss of the two groups (mean ± standard deviation). In the N3PUFA there was statistically significant decreased weight loss compared to CONTROL (p = 0.004).
Measurement of cytokines (IL - 1b, IL -6, and TNFa)
The values of cytokines (IL - 1b, IL -6, and TNFa) in both experimental groups at the start of the experiment were compared and no statistically significant difference was found (p = 0.641, p = 0.381, p = 0.184, respectively). Furthermore, the statistical test, concerning the cytokines, IL-1b, IL -6, and TNF at the sacrifice, did not reveal a statistically significant difference between the two groups (p = 0.279, p = 0.265, and p = 0.254, respectively). According to the Wilcoxon test, there was a statistically significant increase in cytokines levels in both groups between the beginning of the experiment and the time before the sacrifice (p < 0.001). The results of these comparison are shown as a bar graph in figures 2A-C, respectively.

Figure 2 A: comparison ofIL-1b of the two groups at the beginning of the experiment and before sacrifice (mean ± standard deviation). There was no statistically significant difference, but increase in cytokines levels within each subgroup was statistically significant (p < 0.001). B: comparison of IL-6 of the two groups at the beginning of the experiment and before sacrifice (mean ± standard deviation). There was no statistically significant difference, but increase in cytokines levels within each subgroup was statistically significant (p < 0.001). C: comparison of TNF-a of the two groups at the beginning of the experiment and before sacrifice (mean ± standard deviation) There was no statistically significant difference but increase in cytokines levels within each subgroup was statistically significant (p < 0.001).
Histology
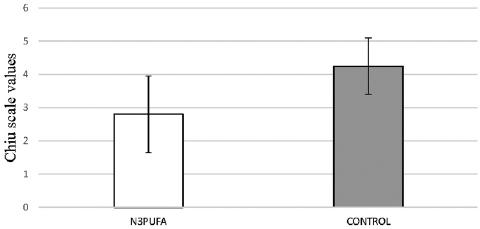
According to Chiu scale, in group N3PUFA, the severity of intestinal ischemia ranged from 1-5, with a mean of 2.8, while in group control, the severity of intestinal ischemia ranged from 3-5, with a mean of 4.3. Statistical control revealed that in Group N3PUFA the mean intestinal gravity value was statistically lower than in group control (p < 0.001). The difference is illustrated as a bar graph in figure 3.
Discussion
Acute mesenteric ischemia is a life-threatening condition with an unfavorable prognosis. The disease is known since 187522, however, during the past decades, its frequency is continuously increasing and affects one case for every 1000 admissions23-25. The causes of the disease include occlusion of the superior mesenteric artery due to embolus in 50% of cases, thrombosis of the superior mesenteric artery (25%) or vein (5%) and non-occlusive intestinal ischemia in 20%23. Various studies have indicated that the causes of the disease are related directly to mortality, for example mesenteric vein thrombosis is considered to have more favorable outcome when compared with a superior mesenteric artery embolus. Most researchers conclude that the diagnosis of the disease in the first 24 h of the onset of symptoms and before the installation of irreversible damage to the intestinal wall dramatically improves survival rates4. The predisposing factors of the disease include age older than 50 in associations with congestive heart failure, arrhythmias, history of a recent heart attack, hypovolemia, hypotension, and sepsis26. Furthermore, high risk is thought to be patients with a history of arterial embolism, vasculitis, and deep venous thrombosis, as well as patients with hypercoagulable states, such as deficiency of protein C and S and antithrombin III. However, acute mesenteric ischemia, also, occurs in younger people with none of the above risk factors4.
The effects of acute mesenteric ischemia in the intestinal wall are the same regardless of the etiology, ranging from simple disturbances of the intestinal function to wall necrosis and peritonitis27. Tissue damage is associated as well with the release of oxygen free radicals during reperfusion. Oxygen free radicals cause cellular damage and damage of the mucosal barrier, promoting in this way bacterial translocation, which is responsible for sepsis and MODS28.
During the past decades, various experimental models have been used in order to study the mechanism of ischemic bowel necrosis. The most commonly used experimental animal is the rat and ischemic necrosis is achieved by mechanical obstruction of the superior mesenteric artery. Initially, occlusion of the superior mesenteric artery was achieved by ligation, but this technique was accompanied by a high mortality rate (8-80% in the first 85 min)29-31. Therefore, scientists realized that the study of ischemic bowel necrosis based on these protocols wasn't reproducible or reliable, especially for testing the effect of certain drugs32. So they introduced protocols that involved partial occlusion of the superior mesenteric artery33.
In this study, we used an experimental model in rats, where we achieved a controlled narrowing of the superior mesenteric artery using a 23 gauge needle (diameter: 0.65 mm). The stenosis resulted in ischemic lesions of the small intestine which were classified based on the scale by Chiu et al.20. Picazo et al., in a similar experiment, observed that, after controlled narrowing of the superior mesenteric artery, larger lesions were found in the terminal ileum which was gradually evolving through time, from simple mucosal lesions during the first 24 h to wall necrosis after 72 h34.
Moreover, in the present study, we observed a statistically significant decrease in body weight of the animals before and after the occlusion of the superior mesenteric artery in both groups. However, the comparison between the two groups revealed no statistically significant difference. Kotani et al. reached the same conclusion in their experimental model19. Based on the previous studies of Jones et al. and Stoney and Reilly, the weight loss in ischemic enteritis is due to abdominal pain that accompanies food intake and leads the animals to avoid feeding35,36.
The inflammatory reaction of acute mesenteric ischemia is accompanied by alterations in the diameter and permeability of the microvascular circulation. Increased vascular permeability leads to the exit of plasma proteins and leukocytes to the interstitium and migration of lymphocyte to the site of the injury37. The major “chemotactic” agent is the bacterial endotoxin (also known as lipopolysaccharide) which is a component of the cell membrane of Gram-negative bacteria and leads to activation of monocytes and macrophages and production of cytokines, such as TNF-a, IL-1, IL-6, IL-8, and eicosanoids such as prostaglandin E238.
Cytokines that are associated with the inflammatory process are IL-1, TNF-a, and TNF-b and IL-8. IL-1 and TNF-a derive from activated macrophages, while TNF-b by activated T lymphocytes. IL-1 and TNF-a are mainly related to effects such as adhesion of leukocytes to the vascular endothelium, production of prostaglandins, reduction of anticoagulant activity, and upregulation of IL-1, IL-6, IL-8, and PDGF39.
Cytokines produced by monocytes and macrophages modulate the response to infection and trauma. Their actions are downregulated by anti-inflammatory cytokines such as IL-10. Although the inflammatory response is a normal and necessary process, the uncontrollable activation can cause serious damage to the organism. High concentrations of TNF-a, IL-1, and IL-6 have been associated with pathological responses that occur in septic shock, acute respiratory failure, rheumatoid arthritis, and inflammatory bowel disease38.
Several studies suggest that the quantity and type of fatty acid intake may influence lymphocyte function. In experimental protocols using diets based on fish oils, there was an alteration of the function of lymphocytes and suppression of the production of pro-inflammatory cytokines by macrophages39. Similar findings were reported in a protocol, wherein healthy volunteers received a diet rich in n-3 PUFA and there was a reduction in the concentration of monocytes and leukocytes and in the production of pro-inflammatory cytokines40.
There are several studies that suggest that the intestine produces large quantities of IL-1, TNF-a, oxygen free radicals and therefore IL- 6 due to ischemia, although the exact pathway is not quite clear. Furthermore, intestinal ischemia leads to a bacterial overload which leads, then, to an increase in IL-6 and TNF-a41. Moore et al. have demonstrated high levels of cytokines in blood samples taken from mesenteric vessels 60-120 min after induction of intestinal ischemia. Therefore, they concluded that high levels of cytokines are early prognostic indicators of acute intestinal ischemia42. Based on these and on similar observations, Karadaac et al. tried to use the measurement of cytokines for the early diagnosis of acute mesenteric ischemia. However, their results did not show statistically significant difference and also the determination of the level of cytokines was time consuming and could not overcome the great challenge of early diagnosis41. In the present study, we measured the level of serum cytokines IL-1b, IL-6, and TNF-a prior and after the occlusion of the superior mesenteric artery in both groups and no statistically significant difference were found. Based on these results, we concluded that the administration of an enriched in n-3 PUFA diet failed to achieve reduction of the level cytokines and thus a possible modification of the SIRS. However, the tissue levels of cytokines were not measured and so a local anti-inflammatory response cannot be ruled out.
It is well known that PUFA play an important role in various biological functions. The long chain n-6 PUFA, and especially arachidonic acid (20: 4n-6), are precursors of eicosanoids and contribute to the production of prostaglandins and leukotrienes43. There are many studies that correlate the consumption of PUFA with the pathogenesis of inflammatory bowel diseases and, in particular, through their involvement in the inflammatory response44,45. In recent decades, the influence of western diet on the inflammatory process has been extensively studied. Typical Western diets include a high ratio of n-6/n-3 PUFA. N-6 PUFA are the precursor of arachidonic acid which is a strong pro-inflammatory agent37,46. On the contrary, n-3 PUFA, which are found in large quantities in fish oils, are considered to have various beneficial properties, particularly in the regulation of inflammatory and immune response. In general, n-3 fatty acids derivatives are thought to activate milder inflammatory and immune responses compared with the arachidonic acid derivatives. For this reason, researchers believe that they probably promote a milder inflammatory response compared to n-647.
As mentioned above, acute mesenteric ischemia inevitably evolves into SIRS and MODS. Sepsis and septic shock are the main causes of death in these patients in intensive care units. The SIRS is triggered by both bacterial translocation and the systemic response to tissue damage48. Many researchers try to evaluate the potential beneficial role of n-3 PUFA. Therefore, they introduced the concept of “immunonutrition” which includes diets with a specific fatty acid composition that could affect the inflammatory and immune response48. In several studies, the use of an enteral diet rich in n-3 fatty acids after a major surgery or injury resulted in reduction of septic complications49. Furthermore, administration of enteral or parenteral diet rich in n-3 fatty acids in septic models resulted in increased survival and reduced respiratory failure50. In this study, we observed a difference in ischemic lesions between the two experimental groups. Specifically, there was a statistically significant reduction in the mean intestinal lesions in the group that received an enteral diet rich in n-3 PUFA. Based on these findings, it is thought that a diet rich in n-3 fatty acids may be beneficial and can significantly reduce the impairment of the intestinal mucosa villi, by modifying the local, but not the systemic, immune, and inflammatory response.
Conclusions
The use of a diet enriched with n-3 PUFA is beneficial by reducing the damage of intestinal mucosa accompanying the acute mesenteric ischemia. N-3 fatty acids healing induction may be caused by modifying the local inflammatory and immune response of the gastrointestinal tract, which was not measured in the current experiment, to intestinal ischemia.











 nova página do texto(beta)
nova página do texto(beta)