Introduction
Acid-base homeostasis is vital for the especially central nervous system. Aydin et al. showed that thermoregulatory corpuscles regulate cerebrospinal fluid and brain temperature1, but pH regulating sensors have not been documented in the brain. The chemoreceptor network consists of carotid body/glossopharyngeal nerve is necessary for blood2 and cerebrospinal fluid pH regulation. However, some pH-sensitive cell extensions discovered in the spinal cord, which regulate cerebrospinal fluid pH3. Acidosis is the most trouble complication of subarachnoid hemorrhage (SAH), as described by Ozmen et al.4. Furthermore, carotid body lesions in spine surgery can cause a decrease in blood pH5. A new cause of spastic disorders could be attributed to central canal hemorrhages6. Although anterior spinal artery7 or Adamkiewicz artery vasospasm8 has been accused of the spinal cord, peripheral nerve complex, and distal organ denervation injuries, we newly discovered that not only ischemic degeneration but also decreased blood and cerebrospinal fluid pH could be responsible for neurodegeneration which has been mentioned so far. The choroid plexuses involved in the production and reabsorption of cerebrospinal fluid9 which regulate brain temperature1 through deposed water vesicles10. If carotid bodies and choroid plexus undergo ischemic insult, then decreased pH/cerebrospinal fluid could cause irreversible dangerous neuronal injury in both brain and spinal cord networks mentioned by in this study.
Material and Methods
This experiment was studied according to the Ethics Committee of Ataturk University.
Animal selection
This experiment was done on 23 hybrid rabbits which five used control (n = 5), six of SHAM injection of 0.5 cc saline solution (n = 6), and the remaining used as SAH group (n = 12) with the injection of 0.5cc of autologous blood into their fourth ventricles. All animals followed 3 weeks and humanely sacrificed under general anesthesia.
Used experiment protocol
Routinely used injectable anesthetics were applied to reduce pain and mortality. Isoflurane (0.2 mL/kg) given by a facial mask with an anesthetic combination (Ketamine HCL, 150 mg/1.5 mL; Xylazine HCL, 30 mg/1.5 mL; and distilled water, 1 mL) injected subcutaneously at the beginning of surgery. Anesthetic combination of 0.1 mL/kg used during surgery if required. Auricular arterial autologous blood (1 mL) injected with a 22-Gauge needle into fourth ventricles to created SAH and saline solution (1 mL) injected to SHAM animals. The animals were sacrificed after 3 weeks and their carotid bodies, brains, cervical spinal cords, and dorsal roots at the level of C-4 were extracted and then kept in 10% formaline solutions. Cervical specimens and carotid bodies longitudinally but midcoronal brain sections embedded in paraffin blocks to observe brain ventricles and central canal to examine histopathological methods. All carotid body and brain tissues were sectioned of five micrometers consecutively but the spinal cord sectioned in numbers of 20-50 sections. They were stained with H and E and GFAP methods. The Cavalieri method was used to evaluate the volumes of the central canal. A number of consecutive sections obtained from tissue samples of spinal cords arranged over and over to obtain 3D appearances of the central canal as same as cylinders. All central canals were accepted as cylinder and their volumes were calculated as the cylinder methods using the required formula. To estimate the volume of their photographs were taken on the prepared forms of glass lamellae and surface areas of the central canal were calculated using mini squared papers described by Yolas et al.11.
Main results
Cardiac and respiratory arrhythmias, unconsciousness, simple convulsions, and neurological reflex abnormalities occurred in SAH-created animals. Three animals dead in the SAH group. Subarachnoidal inflammation, adhesion, blood collection, congested spinal cord vessels, and contracted anterior spinal artery observed in the SAH group in postmortem examinations.
Histopathological results
To estimate the ependymal cell density of the central canal surfaces of the spinal cord, central canal volumes, all cervical spinal cords were sectioned coronally at the levels of the biggest part of intumescentia cervicalis (Fig. 1A). For the central canal volume and ependymal cell estimation, longitudinal spinal cord sections were done at the mentioned levels (Fig. 1A). Histopathological appearances of central canal surfaces, choroid plexuses, and ventricles are seen in Figures 1 and 2 shows degenerated neurons of the carotid body, normal choroid plexus with water vesicles, and deformed/degenerated choroid plexus, deformed/degenerated ependymal cells are seen in a SAH created rabbit which owned low pH values. Transverse section of the spinal cord with central canal second motor neurons normal ependymal cells deformed degenerated ependymal cells and normal/deformed degenerated second motor neuron in the gray matter of low pH detected animal with SAH (Fig. 3). The findings of cervical spinal cords and dorsal roots at the level of C-4 of all animals resembled as our previous studies mentioned in the presented study at the level of lumbosacral regions12. Furthermore, carotid body examinations resembled that Ozmen et al.4.
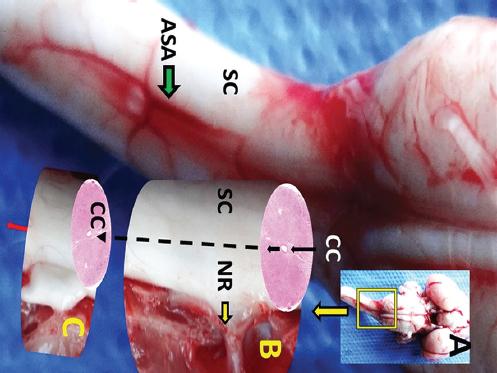
Figure 1 A: Macroscopical appearance of brain with cervical spinal cord (SC) and anterior spinal artery (ASA). B: magnified form of examined spinal cord part with nerve root (NR) of C-4. C: representative extension of spinal cord to shown central canal (CC) localization.
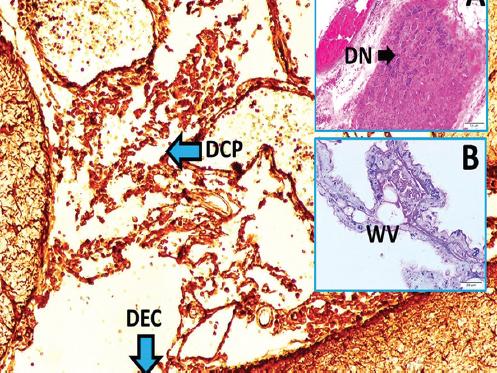
Figure 2 Degenerated neurons (DN) of carotid body (LM, H&E, × 10/A), normal choroid plexus with water vesicles (WV) (LM, H&E, × 40/B), and deformed/degenerated choroid plexus (DCP), deformed/degenerated ependymal cells (DEC) are seen in a subarachnoid hemorrhage created rabbit which owned low pH values (LM, GFAP, × 10/Base).
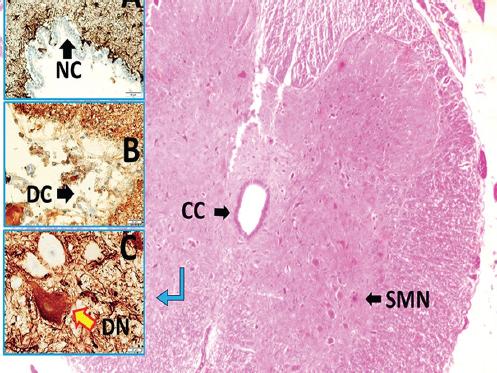
Figure 3 Transverse section of spinal cord with CC, SMN, (LM, H&E, x4/Base), normal ependymal cells (NC) (LM, GFAP, × 20/A), deformed degenerated ependymal cells (DC) (LM, GFAP, × 40/B), and normal/deformed degenerated second motor neuron (DN) in gray matter of low pH detected animal with subarachnoid hemorrhage (LM, GFAP, × 40/C). CC: central canal; SMN: second motor neurons.
The data were analyzed with the software package (SPSS® for Windows v. 12.0, Chicago, USA). The data analysis consisted of the Kruskal-Wallis and Mann-Whitney U tests. Differences were considered to be significant at p < 0.05. Cardiorespiratory disturbances cause 3 animals to exist in the SAH group. Subarachnoidal inflammation, adhesion, blood collection, congested spinal cord vessels, and contracted anterior spinal artery observed in the SAH group.
Numerical results
Mean cervical central canal volume was 1.056 ± 0.053 mm3 and degenerated ependymal cells density was 6 ± 2 in control animals (n = 5); 1.321 ± 0.12 mm3 and 35 ± 9 in SHAM animals (n = 6) and 1.743 ± 0.245 mm3 and 159 ± 24/mm2 in low pH detected animals (n = 6). The mean cerebrospinal pH values were: 7.342 ± 0.034 in the control group; 7.314 ± 0.056 in SHAM, and 7.257 ± 0.049 in the SAH group. Low pH values cause the more ependymal cells desquamation (p < 0.0005) with a direct linear link of degenerated carotid body neuron density (p < 0.0001).
Discussion
For life, acid-base balance is vital. Respiratory and renal systems are essential for pH control3. Acidosis is the most dangerous complication of SAH7. Glossopharyngeal nerve and carotid body web are known as chemoreceptors complex which essential for pH regulation. There is an inverse link between degenerated neuron numbers of carotid body and pH values2. Although the carotid body network is essential for pH regulation and neuronal degeneration of the carotid body could result in acidosis following SAH4, there is no information intracranial pH regulating cells. However, some pH sensors included neuronal networks discovered in the spinal cord, regulate cerebrospinal fluid pH3. A plenty of degenerated neuron density of the carotid body resulted in decreased blood pH and respiratory acidosis5.
The spinal arteries are innervated by vasodilatory dorsal root ganglia. SAH-induced spinal cord arteries vasospasm can be prevented by cervical dorsal root ganglions. Unfortunately, degenerated neuron density of cervical dorsal root ganglions causes severe anterior spinal arteries vasospasm in SAH7. Adamkiewicz artery vasospasm is responsible for both spinal cord and peripheral nerve insult8 and abdominopelvic organs insufficiency13. Adamkiewicz artery spasm causes Onuf's nucleus linked pudendal nerve complex degeneration to also cause urinary retention11 and gastrointestinal functional problems14. Caglar et al. discovered that Onuf's nucleus ischemia-related sacral parasympathetic network insult should be considered as Hirschsprung like disease12.
Spastic disorders have been considered as cerebral complications of SAH, but a recent study showed that de-synchronization of the flexor-extensor muscles innervating interneuronal structures is the uninvestigated agent following the central canal hemorrhage6. In the same manner, ciliospinal sympathetic center ischemia-induced neurodegeneration in the spinal cord can be responsible for permanent miosis following SAH15. Spinal cord tumors may have the same effect16. The irrigation of subarachnoid spaces may decrease the percentage and severity of inflammatory complications by way of the excretion of inflamed purulent collection from the subarachnoid spaces17.
Furthermore, choroid plexuses are the significant foundation for cerebrospinal fluid in which cerebral immune surveillance, various endocrine-enzymatic activities, and cerebrospinal fluid-blood barrier functions impracticable unless choroid plexus. Hence, there may be a significant link between choroid plexus and cerebrospinal fluid pH regulation. SAH may extend to brain ventricles and causes ischemic degeneration of the choroid plexus by way of triggered choroidal artery vasospasm9.
Conclusion
It should not be forgotten that the prevention of choroid plexus/functions can be inevitable for cerebrospinal fluid pH regulation.
Future ınsight
In that article insight, we postulated that cerebrospinal fluid exchange could be used for cerebrospinal fluid pH restoration. In our study, systemic effects of acidosis were found to be irreversible. In the case of acidosis, cerebrospinal fluid exchange should be among the treatment approaches to obtain normal pH, provided that the clinical situation is considered.











 nueva página del texto (beta)
nueva página del texto (beta)


