Introduction
Frantz-Gruber tumor, also known as solid cystic tumor of the pancreas or pseudopapillary tumor, is a rare entity1, which represents 0.7-2.7% of all pancreatic tumors. It’s more frequently observed between the 2nd and 4th decades of life and is more frequent in women2.
The etiology is unknown, however, its distribution by age and sex is oriented to genetic and hormonal factors. The association of this disease with the use of oral contraceptives has not been demonstrated. The association of this disease with the use of oral contraceptives has not been demonstrated3,4.
Initial symptoms are non-specific and a high percentage of them correspond to incidental findings.
The imaging work-up includes computed tomography (CT) and magnetic resonance imaging (MRI) that reveal solid cystic lesion well defined in the head or body of the pancreas5.
Histological confirmation is carried out with markers for vimentin, alpha-1-antitrypsin, CD-10, non-specific enolase, CD-56, and progesterone receptor (PR)6. The overall prognosis is good, with a 5-10 years survival greater than 90%7. Metastases have been reported in 10-15% of cases, most frequently to the peritoneum, greater omentum, liver, and regional lymph nodes. In the absence of metastasis, the main treatment is surgical resection4-8.
We present six cases of Frantz tumor treated by surgical resection in a third level hospital of our environment.
Clinical cases
Case 1
A 53-year-old female patient, with a history of hypertension, dyslipidemia, and hypothyroidism under medical treatment, came in with a 27-day history of epigastric pain, with elevation of liver enzymes (AST 169, ALT 491, and GGT 348), without cholestasis, tumor markers were Ca 19-92.2 U/ml. MRI reported a 3 cm diameter, nodular, regular lesion on the head of the pancreas with cystic areas, without vascular compromise or metastases (Table 1).
Table 1 Table describing the cases
| Cases | Symptoms | Imaging | Treatment | Immunohistochemistry | Evolution |
|---|---|---|---|---|---|
| 1 | Epigastric pain and elevation of liver enzymes without cholestasis | MRI: 3 cm lesion in the head of the pancreas | Open pancreaticoduodenectomy | Alfa-1-antitripsin, Vimentin, CD-10, CD-56, and progesterone receptor + | 34 months follow-up without recurrence at CT |
| 2 | Lumbar pain Incidental finding | CT: 10 cm lesión in páncreas body with áreas of necrosis | Laparoscopic distal pancreatectomy with splenic preservation | Alfa-1-antitripsin, CD-10, CD-56, and progesterone receptor + | 30 months follow-up without recurrence at CT |
| 3 | Acute pancreatitis | CT: heterogenic solid nodular image of 43 mm located in the body of the pancreas | Laparoscopic distal pancreatectomy with splenic preservation | CD 10, CD 56, and progesterone receptor + | 24 months follow-up without recurrence |
| 4 | Palpable epigastric tumor | CT: solid cystic lesion in the head of the pancreas | Open splenopancreatectomy | Alfa-1-antitripsin, CD-10, CD-56, and progesterone receptor + | 20 months follow-up without recurrence |
| 5 | Epigastric pain | CT: lesion in the head of the pancreas | Open pancreaticoduodenectomy | Alfa-1-antitripsin, CD-10, CD-56, and progesterone receptor + | Hepaticojejunostomy stenosis, requiring
percutaneous dilatation. Stump pancreatitis at 24 months, without recurrence. |
| 6 | Incidental finding | CT: hypodense nodule in the head of the pancreas | Open pancreaticoduodenectomy | Alfa-1-antitripsin, CD-10, CD-56, and progesterone receptor + | 21 months follow-up without recurrence at CT |
A pancreaticoduodenectomy with pyloric preservation was performed (Fig. 1). Hospital discharge was given on post-operative day 10 without complications. The histopathological analysis reported solid pseudopapillary tumor of 32 × 28 × 15 mm, with free margins, seven lymph nodes without metastases. Inmunohistochemistry was positive for, alpha-1-antitrypsin, CD-10, CD-56, and PR. Last control at 34 months with CT without recurrence.
Case 2
A 16-year-old female patient, with a complaint of lower back pain. Ultrasound reveals an incidental lesion in the body of the pancreas. A CT was requested that confirmed a 10 cm lesion of the body of the pancreas, of solid appearance with areas of necrosis. CEA and CA 19-9 values were non-remarkable. A laparoscopic distal pancreatectomy with splenic preservation was performed. Discharge was given on post-operative day 4, without any complications. The pathology report was positive for a pseudopapillary neoplasm of 85 × 90 mm, with free surgical margins (Fig. 2). The last control of the patient was at 34 months with CT that did not show recurrence.
Case 3
An 18-year-old female patient with a history of acute pancreatitis of biliary origin was found to have a heterogeneous solid nodular image of 43 mm in diameter located in the middle segment of the pancreas in an abdominal ultrasound and CT, tumor markers were CEA 0.8 U/ml and Ca 19-93.0 U/ml. A laparoscopic distal pancreatectomy with splenic preservation was performed, using the Warshaw technique. The biopsy reported solid pseudopapillary neoplasm of 50 × 45 × 30 mm, free surgical margins, positive CD 10, CD56, and PR. Discharge was given at day 8. Follow up at 30 months did not show recurrence.
Case 4
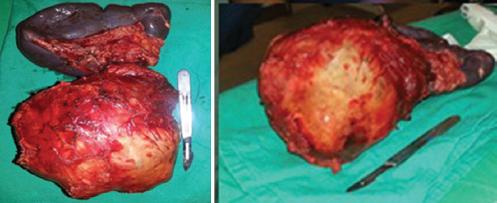
A 14-year-old female patient, otherwise healthy, consulted for a palpable non-painful abdominal mass. The CT showed a 10 cm solid cystic lesion in the body of the pancreas, without metastases tumor markers were CA 19-92.8 U/ml. Open distal pancreatectomy without splenic preservation was performed (Fig. 3A and B). The pathology report confirmed a 116 × 130 × 75 mm, pseudopapillary neoplasm compatible with Frantz tumor with free surgical margins, positive alpha-1-antitrypsin, CD-10, CD-56, and PR. Control at 20 months without recurrence.
Case 5
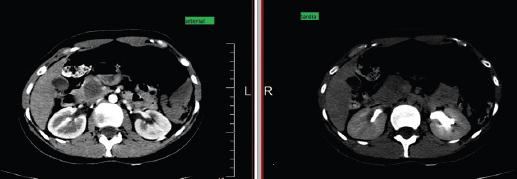
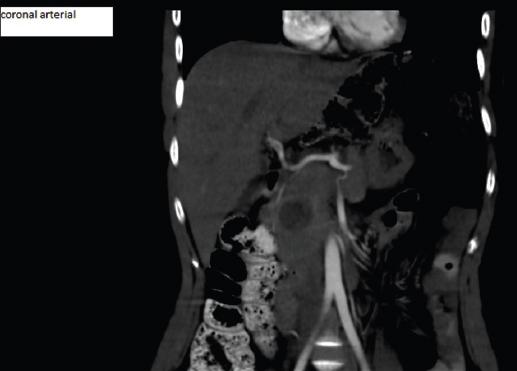
A 23-year-old female patient, previously healthy, consulted for epigastric pain tumor markers were CA 19-93.2 U/ml. CT showed solid cystic lesion in the head of pancreas, without infiltration of the bile duct, Wirsung duct, or mesenteric vessels, neither evidence of metastases (Fig. 4-5). An open pancreatoduodenectomy was performed. Histopathological analysis confirmed a solid pancreatic pseudopapillary neoplasm 26 × 25 mm, positive alpha-1-antitrypsin, CD-10, CD-56, and PR. At the first month of follow up after surgery, labs showed elevated liver enzymes, abdominal ultrasound showed dilation of the intra hepatic bile duct, secondary to stenosis of the hepatijejuno-anastomoses, confirmed by MRI. A percutaneous dilatation of the stenosis was performed. At 20 months, the patient presented with an episode of mild acute pancreatitis Balthazar A, without relapse of disease.
Case 6
In a surveillance CT of a 34-year-old female with a history of breast cancer treated with surgery and chemoradiation, a 20 × 18 cystic lesion was found in the head of the pancreas without extraglandular involvement a hypodense, 20 × 18 mm, nodular lesion with cystic components located in the head of the pancreas, without extraglandular involvement was found. These findings were confirmed by MRI. Laboratory was non remarkable, and CA 19-9 was 9 U/ml. A pancreaticoduodenectomy was performed. The pathology report confirmed a solid pseudopapillary pancreatic neoplasm, free margins, positive alpha-1-antitrypsin, CD-10, CD-56, and PR. The patient was discharged on day 7 without complications. No relapse was recorded at 21-month follow-up.
Discussion
Solid pseudopapillary tumor of the pancreas is a rare entity that accounts for about 1% of all pancreatic tumors, being more frequent in women (W/M 10:1) between the 2nd and 4th decades of life, although up to 20% of cases can occur in pediatric patients1-8.
Although it can reach sizes of more than 10 cm and invade adjacent structures, it is a tumor with a good prognosis and the occurrence of metastasis is rare. Malignancy should be suspected in the presence of imaging signs suggestive of vascular, nervous, lymphatic, or hepatic metastasis9. In our experience, no metastases were recorded in our experience.
These are slow-growing tumors and their clinical presentation is varied, vague and non-specific. In our experience, 50% of the cases were incidental imaging findings. The mass effect can generate symptoms of compression, abdominal pain, and feeling of fullness. Isolated cases of rupture of the capsule with hemoperitoneum and acute abdomen have been described10. In cases located in the head of the pancreas, biliary obstruction is very rare, unlike adenocarcinomas.
The most frequent location is the head of the pancreas (30-40%), followed by a frequency of 32% and 28% in body and tail, respectively1-8. The treatment must be adapted to the tumor location, surgical risk, and the possibility of achieving complete resection (R0). In our series, we presented three cases in the head of the pancreas and three in the body, then three pancreaticoduodenectomies and three distal pancreatectomies were performed.
Regarding additional tests, the laboratory does not usually show alterations and the tumor markers are rarely altered11. Abdominal ultrasound is the first-line diagnostic method because it is readily accessible and minimally invasive, with no need to irradiate the patient. It allows the identification of lesions in solid organs, often incidentally12, however, it must be complemented with CT and MRI, which are the preferred methods in case of suspected diagnosis, due to the characteristics of the tumor and the need of evaluation of the vascular invasion. On CT, an encapsulated mass with solid cystic components can be observed due to necrohemorrhagic degeneration, as well as calcifications in its periphery13. On the other hand, in nuclear magnetic resonance, it can be seen as a well-defined tumor with solid isointense areas with the pancreas in T1 or a signal slightly high in T2. The cystic areas appear as high-intensity signals in T114.
It is very important to consider pancreatic pseudocysts as a differential diagnosis, since 60% of these resolve spontaneously. In the presence of a suspicious lesion and in the absence of a history of acute pancreatitis, it is convenient to consider it as a cystic neoplasm, without delaying the surgical intervention15.
Regarding surgery, the laparoscopic approach is valid and also facilitates splenic preservation in tumors located in the body and/or tail. In a comparative study between laparoscopic distal pancreatectomy versus the open technique, it was demonstrated that the laparoscopic technique is associated with less blood loss, a better post-operative recovery in terms of shorter hospital stay, lower risk of general postoperative complications, and infection of the surgical wound16. Our experience is consistent with the literature in this regard.
Among the techniques of splenic preservation, Kimura’s technique consists of maintaining the integrity of the anatomy of the splenic artery and vein, whether Warshaw’s allows the vascular viability of the spleen in cases of splenic artery and vein ligation through the integrity of the short gastric and gastroepiploic vessels in their paths into the splenic hilum17. The preservation of the spleen, using the Warshaw technique, allows a pancreatic resection surgery with a significantly shorter operative time and blood losses significantly lower than those of the technique that tries to maintain the integrity of the splenic vessels18. Ten retrospective studies involving 699 patients showed that the Warshaw technique was associated with a shorter operative time (p < 0.0001). There were no differences in blood loss (p < 0.45). However, the appearance of gastric varices and splenic infarction was significantly higher in the group of the Warshaw techniqu17-19. In our experience, both techniques showed good results.
Splenic preservation is not free of complications as portal thrombosis, infarction, splenic abscess, and hemorrhage have been reported18.
Other differential diagnoses are endocrine tumors, acinar cell carcinoma, pancreatoblastoma, ductal adenocarcinoma, and cystic tumors of the pancreas. Differential intraoperative diagnosis is often difficult, so immunohistochemical studies in pathological anatomy results are very important20.
The immunophenotype of this neoplasm is usually positive for vimentin > 90%, alpha-1-antitrypsin 90%, > 80% nonspecific enolase, CD-56, and PR. About 75% can express positivity for cyclin D120,21. In our six cases, 100% were positive for CD-10, CD 56, and PR, 83.3% were positive for alpha-1-antitrypsin and only 1 case (16.6%) was positive for vimentin.
In the results of pathology reports, venous invasion, perineural invasion, high nuclear grade, nests of necrotic tissue, and capsular invasion are factors of high malignant potential15.
The prognosis of these patients is considerably better than that of patients with equal invasion, but with a pancreatic tumor of another histological type. Resection is indicated even in the presence of liver metastases, since it has high survival aggressive management should always be used in patients in whom this type of tumor is suspected even when there is invasion of neighboring structures, or metastasi19. The estimated survival at 1.3 and 5 years is 99.4%, 97.5%, and 96.9%, respectively13. The size of the tumor should not be a factor of unresectability.
Conclusion
Frantz tumor is a rare pancreatic neoplasm. Imaging findings are suggestive, but diagnostic confirmation is provided by histopathology and immunohistochemistry. They have a low malignant potential, and the gold standard of treatment is surgical excision ensuring free margins.
The laparoscopic approach is a safe and effective way of performing pancreatic surgery for the treatment of the left pancreas tumors. Both techniques SVP and WT are safe and feasible to preserve the spleen, although there is no significant benefit of one technique over the other. The choice between them must be made according to each case.











 nova página do texto(beta)
nova página do texto(beta)