Introduction
Olfactory neuroblastoma (ONB) is a rare malignant neoplasm that arises at the upper nasal cavity and represents 3-5% of all sinonasal malignancies1,2. The most common symptoms are nasal obstruction, recurrent epistaxis, headache, facial pain, sinusitis, and anosmia. The advanced disease presents with diplopia, proptosis, decreased visual acuity, frontal lobe syndromes, and seizures1. ONB exhibits variable biological and clinical behavior, ranging from indolent to highly aggressive tumor with the potential of regional and distant metastases. Given the anatomic location and rarity of ONB, it has been difficult to determine the optimal treatment strategy. A multimodal approach is recommended as most of the cases present at an advanced stage. Complete surgical resection followed by post-operative radiotherapy is the standard of care and provides the best outcome in terms of survival and recurrence1.
Materials and methods
We completed a retrospective analysis of patients with ONB treated at the National Cancer Institute, Mexico City from January 2011 to January 2018; a total of 12 cases were identified. Patients records were reviewed for demographic and clinical data including debut symptoms, modified Kadish stage, Hyams grade, surgical approach (transcranial or endoscopic), extent of resection, complications of treatment (surgery and radiotherapy), functional status according to Karnofsky scale (KPS), recurrence, follow-up, and overall survival.
The local Institutional Review Board (Ethics and Research Committees) approved reviewing the medical files of the patients.
Results
We found 12 patients with the diagnosis of ONB (Table 1), 11 males (92%) and 1 female (8%) with a mean age of 48 years. The most common symptoms were nasal obstruction in 10 cases (83%), craniofacial pain in 7 cases (58%), and epistaxis in 7 cases (58%). According to the modified Kadish staging, the proportion of B, C, and D stage was 16%, 58%. or 25%, respectively. Regarding Hyams classification, the proportion of Hyams 1, 2, or 3 was 25%, 50%. or 25%, respectively. Surgery was the mainstay treatment in all cases. The most common surgical approach was craniofacial in 5 cases (42%), followed by the transfacial in 4 cases (33%) and the endonasal endoscopic approach in 3 cases (25%).
Table 1 Demographic data
| Mean age (SD) | 48.8 (10.9) |
| Gender (%) | |
| Male | 11 (92) |
| Female | 1 (8) |
| Symptoms (%) | |
| Nasal obstruction | 10 (83) |
| Local pain | 7 (58) |
| Epistaxis | 7 (58) |
| Kadishstage (%) | A n = 0 (0) B n = 2 (17) C n = 7 (58) D n = 3 (25) |
| Hyams grade (%) | 1 n = 3 (25) 2 n = 6 (50) 3 n = 3 (25) |
| Surgicalapproach | |
| Firstsurgery: (%) | |
| Craniofacial | n = 5 (42) |
| Transfacial | n = 4 (33) |
| Endoscopic | n = 3 (25) |
| Secondsurgery: (%) | |
| Endoscopic | n = 3 (60) |
| Transfacial | n = 2 (40) |
| Gross total resection | 8 (67%) |
| Subtotal resection | 4 (33%) |
| Radiotherapydose (%) | |
| 60 Gy in 30 Fx | 3 (25) |
| 30 Gy in 10 Fx | 3 (25) |
| Other (range 45-70Gy) in 25-35Fx | 6 (50) |
| Chemotherapy | |
| Cisplatin | n = 2 (16%) |
| Median KPS pre-operative | 95 |
| Median KPS post-operative | 90 |
| Median painventilator-associatedevent pre-operative | 5.5 |
| Median painventilator-associatedevent post-operative | 2.5 |
| Mortality | 1 (8%) |
| Mean survival (SD) months | 63.6 (50) |
| Mean follow-up (SD) months | 41.1 (38) |
KPS: Karnofskyscale.
In terms of surgical resection, the craniofacial approach achieved gross total resection (GTR) in 4 patients (80%) and subtotal resection (STR) in 2 patients (20%). The transfacial group had GTR in 3 patients (66%) and STR in 1 patient (33%), while the endoscopic endonasal group achieved GTR in 1 patient (33%) and STR in 2 patients (66%) (Figs. 1-4).

Figure 1 Pre-operative sagittal and coronal T1-weighted imaging of olfactory neuroblastoma in the nasal cavity invading anterior fossa through lamina cribosa.
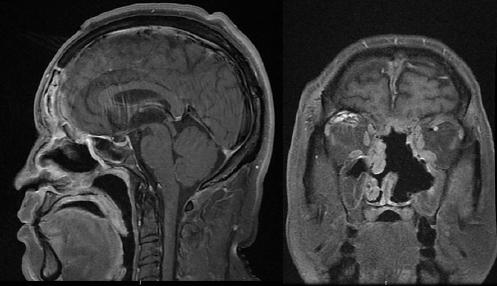
Figure 2 Post-operative magnetic resonance imaging T1-weighted imaging of the case in figure 1. A combined cranionasal approach was performed to achieve resection.
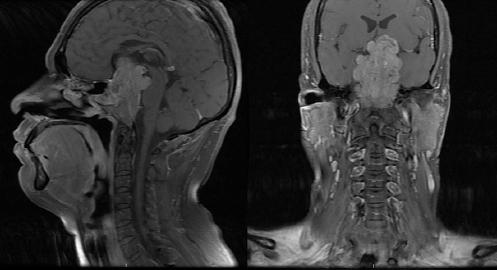
Figure 3 Pre-operative sagittal and coronal magnetic resonance imaging T1-weighted imaging of an unusual case of olfactory neuroblastoma with intra, supra, and retrosellar extension.
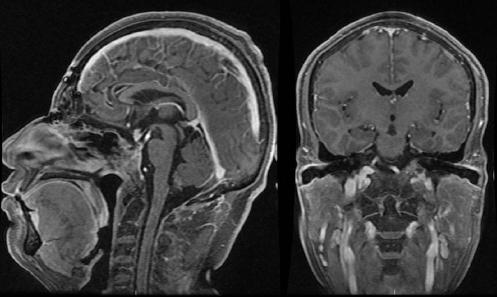
Figure 4 Post-operative sagittal and coronal magnetic resonance imaging T1-weighted imaging T1WI of figure 3. A combined transcranial and endonasal approach was used to achieve complete surgical resection.
After surgery, all patients received radiotherapy ranging from 30 to 70 Gy in 10 to 32 sessions, depending on the clinical response.
Five patients (42%) underwent a second operation due to recurrent/progressive disease. Three patients (66%) were re-operated through endoscopic endonasal approach and 2 cases (33%) with the transfacial approach.
Cisplatin, the most commonly used chemotherapy regimen, was used in 2 patients with recurrent disease.
We documented metastases in 9 cases: one to sagittal sinus3, frontal and parietal lobes (Fig. 5), 1 to the parotid gland, 1 to the left orbit, and 6 had cervical ganglia metastases. None of the neck nodes metastases was resected and instead received radiotherapy.

Figure 5 Distant meningeal metastases of olfactory neuroblastoma through the superior sagittal sinus.
In terms of surgical complications, there was 1 patient with frontal lobe syndrome after surgery, and 3 patients with transient diminished visual acuity and diplopia with full recovery at follow-up. Our surgical complication rate was 8.3%. Progression-free survival was 41 months, like other literature reports4-11. The mean survival was 63.6 months (Fig. 6).
Post-operative KPS is not usually reported in the ONB series. In our series, KPS improved in 4 patients (40%), did not change in 4 patients (40%), and worsened in 3 patients (30%). One patient (8%) died due to progression of the disease.
In general terms, the results of this retrospective clinical analysis (Table 1) are consistent with what has been reported in other series of ONB cases (Table 2).
Table 2 Other series of cases of olfactory neuroblastoma reported in the literature
| Series | Patients (n) | Tumor-node-metastasis | HYAMS | Kadish | RT/Chemo | Approach | Follow-up (months) | Progression-free survival | OS | Extent of resection | Mortality | Complications | KPS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nakagawa et al.4 | 22 | T1: 27% T2: 41% T3: 23% T4: 9% |
I: 9% II: 86.5% III: 4.5% |
A: 18% B: 23% C: 59% |
91% RT | Endoscopic | 11-104 Mean 44 | 95.5% at 5 years | NR | 95.5% GTR 21 of 22 patients | No | 10% cerebrospinal fluid leaks | NR |
| Rimmer et al.5 | 95 | T1: 27% T2: 28.4% T3: 11.6% T4: 32.6 |
NR | A: 72% B: 59% C: 47% |
RT: 32.6% chemo 33.6% | 65 Craniofacial 30 Endoscopic | 1-309 Mean 88.6 | 80% at 5 years and 62.8% at 10 years | 83.4% at 5 years and 76.1 at 10 years | NR | 21 | NR | NR |
| Nalavenkata et al.6 | 113 | NR | I: 6.2% II: 31.9% III: 28.3% IV: 5.3% indeterminate: 28.3% |
A: 9.7% B: 26.5% C: 63.5% |
RT: 72.5% | Endoscopic 59.3% patients Endoscopic assisted 16.8% open craniofacial 20.4% | 41.5 Median | 92.4% at 5 years and 79.6% at 10 years | 205 months | 70.8 % GTR 24.8 STR | NR | 3.5% Postoperativeinfections, 4.4 % neurologic deficits, 1.8 epistaxis, 7.1% cerebrospinal fluid leaks | NR |
| Bell et al.7 | 124 | T1/T2: 14% T3: 16% T4: 52% |
I/II: 62% III/IV: 21% | A: 16% B: 33% C: 43% D: 8% |
RT: 76% Chemo: 36% | Endoscopic: 32% craniofacial: 68% | 63 | 60% at 5 years | 75% at 5 years | 56% GTR | NR | NR | NR |
| Yuan et al.8 | 44 | T1: 13.6% T2: 34.1% T3: 29.5% T4: 22.7% |
NR | A: 6.8% B: 36.4% C: 27.3% D: 29.5% |
RT: 70.4% Chemo: 58.9 % | No surgery: 47.7% open craniofacial: 20.5% endoscopic assisted: 11.4% endoscopic: 22.6% | 27-198 median 84 | 39.1% at 5 years and 21.7% at 10 years | 42.7 at 5 years and 28% at 10 years | NR | NR | NR | 90-100: 31.8% 80-90: 68.2% (preoperatory) |
| Bartel et al.9 | 9 | NR | I/II: 56& III/IV: 44% | A: 11% B: 22% C: 66.7% |
RT: 100% Chemo: 44.4% | endoscopic: 44.4% combined: 44.4% craniofacial: 11.1% | 10-107 mean 52.5 | 88.9% at 5 years | 88.9% at 5 years | NR | 1 died at 40 months | NR | NR |
| Banuchi et al.10 | 57 | NR | NR | A: 23% B 35% C: 33% D: 9% |
RT: 75% | Surgery: 92% open: 77% endoscopic assisted: 11% endoscopic: 8% craniotomy: 4% | 68 | ForKadish A: 69%, B: 56%, C: 46%, D: 0% at 10 years |
85% at 5 years and 75% at 10 years | NR | NR | NR | NR |
| Xiong et al.11 | 187 | NR | NR | A: 12.3% B: 25.7% C: 60.4% |
RT: 81.8% Chemo: 19.8% | Surgery: 79% | 1-204 mean 81 | 57.5% at 3 years | 66.7% at 3 years | GTR: 77.4% STR: 22.6% | NR | NR | NR |
GTR: gross total resection; KPS: Karnofsky scale; STR: subtotal resection; RT: radiation therapy; NR: no reported.
Discussion
ONB is a rare malignant neoplasm that was first described in 1924 by Berger, Luc, and Richard, and given the name esthésioneuroépithéliome olfactif12. ONB is believed to originate from the cribriform plate, the Jacobson's organ (vomeronasal organ), sphenopalatine ganglion, olfactory placodes, and the Loci´s ganglion (nervus terminalis)13; nonetheless, the exact histogenesis of this tumor is not clearly defined1,14.
Diagnostic workup
After a clinical suspicion is raised, the imaging routine should include both a high-resolution contrast-enhanced computed tomography (CT) scan of the paranasal sinuses and neck and contrast-enhanced magnetic resonance imaging of the head and paranasal sinuses. The former is for evaluation of bony erosion of the orbit, skull base, and cervical lymph node involvement; the latter is best suited to delineate orbital, dural, and intracranial extension. Given that differential diagnosis of ONB from non-ONB tumors can be challenging, tissue diagnosis is essential.
Histology
ONB has a lobular architecture composed of small, round blue cells with high nuclear to cytoplasmic ratio, hyperchromatic chromatin, and rare nucleoli. True neural rosettes (Flexner-Wintersteiner) may be observed, while pseudorosettes (Homer Wright) are present in 30% of cases1,15. On the immunohistochemistry staining, this tumor demonstrates diffuse positivity with neuron-specific enolase, synaptophysin, chromogranin, Class III beta-tubulin and EPCAM, and variable S-100 positivity and negative FLI-1 which rule out the diagnosis of peripheral neuroectodermal tumor/Ewing sarcoma; the Ki-67 reveals a moderate to high proliferation index of 10-50%1.
Tumors commonly confused with ONB include sinonasal under differentiated carcinoma, sinonasal neuroendocrine carcinoma, small cell carcinoma, Ewing sarcoma/PNET, pituitary adenoma, melanoma, paraganglioma, lymphoma, and rhabdomyosarcoma15.
Grading
In 1988, Hyams et al.16 developed a grading system which stratifies ONB into four groups from well-differentiated (Grade I) to the least differentiated (Grade IV) based on mitotic activity, nuclear pleomorphism, rosette formation, necrosis, disorganized architecture, and sparse fibrillary matrix (Table 3). It is a complex and subjective system and the distinction between grades is arbitrary; therefore, there is a tendency to group these categories into a low grade (Grade I/II) and high Grade (III/IV)1. There has been growing evidence that Hyams grading correlates with the outcome of ONB and can be used as a prognostic indicator and guide the selection of adjuvant therapies1.
Table 3 Hyams histopathological grading16
| Grade | Lobular architecture preservation | Mitoticindex | Nuclear pleomorphism | Fibrillary matrix | Rosettes | Necrosis |
|---|---|---|---|---|---|---|
| I | + | None | None | Prominent | HW | None |
| II | + | Low | Moderate | Present | HW | None |
| III | +/− | Moderate | Prominent | Low | FW | Rare |
| IV | +/− | High | Marked | Absent | None | Frequent |
HW: homer wright rosettes; FW: Flexner-Wintersteiner rosettes.
Staging
The Kadish classification17 was the first staging system and is popular given its simplicity and ease of application: a tumor limited to the nasal cavity, B involvement of the paranasal sinuses, and C extension beyond the paranasal sinuses. In 1993, Morita et al.18 proposed a modification by adding Stage D for those with cervical lymph node or distant metastases.
Dulguerov and Calcaterra19 proposed a tumor-node-metastasis staging system (also known as the University of California, Los Angeles staging system) with a more detailed description of tumor extension, lymph node, and distant metastases (Table 4).
Table 4 Dulguerov-Calcaterra tumor-node-metastasis staging system19
| Stage | Characteristics |
|---|---|
| T1 | Tumor involving the nasal cavity and/or paranasal sinuses (excluding sphenoid), sparing the most superior ethmoidal cells |
| T2 | Tumor involving the nasal cavity and/or paranasal sinuses (including the sphenoid), with extension to or erosion of the cribriform plate |
| T3 | Tumor extending into the orbit or protruding into the anterior cranial fossa |
| T4 | Tumor involving the brain |
| N0 | No cervical lymph node metastases |
| N1 | Presence of cervical lymph node metastases |
| M0 | No metastases |
| M1 | Metastases |
Treatment modalities
Surgery
The mainstay of treatment is surgery. It provides proper tissue samples for histopathological diagnosis and prognosis and relief of compression symptoms. Various surgical approaches are possible, with the traditional standard of care being an anterior craniofacial approach that comprises lateral rhinotomy, midfacial degloving, or Weber-Ferguson incision with maxillectomy, and/or sinusotomies, along with a bifrontal craniotomy. After resection, a vascularized anterior pedicled pericranial flap can be harvested to re-establish de division between the intranasal and intracranial spaces2. A 2012 international cooperative study of 17 centers reported a 5-year overall survival of 78% and a 5-year recurrence free-survival of 64% with craniofacial resection20.
In cases of more extensive local invasion, endoscopic techniques may also complement transcranial approaches in view of optimizing oncologic margins. Recently, the combined cranionasal approach incorporates endonasal endoscopic resection of accessible portions of the tumor from below with a bifrontal craniotomy to address areas of significant intracranial pathology, with the avoidance of a facial incision2. Komotar et al.21 made a systematic review comparing open, endoscopic, and endoscopically assisted approaches. The endoscopically assisted group demonstrated a higher proportion of GTR s (100 vs. 85%) and negative margins (95 vs. 77%), a lower incidence of local recurrence (16 vs. 22%), and a higher incidence of disease free survival (81 vs. 61%) compared with the open group. Nonetheless, the open group had a longer median follow-up time compared with the endoscopically assisted group, which may have influenced reports of relapse-free survival rates.
Description of techniques and approaches varies among literature, but the overall concept is a systematic dissection of all adjacent paranasal sinuses thereby providing wide surgical exposure of the tumor pedicle to the anterior skull base allowing for complete resection with oncologic margins. Surgery entails the risk of pneumatocele, cerebral edema, cerebrospinal fluid leak, meningitis, cerebral abscess, and cognitive sequelae due to frontal lobe syndrome. Regarding purely endoscopic endonasal approaches, one must consider its limits: spread of the tumor inside the frontal sinuses, invasion of the dura laterally and above the orbits, and invasion of the craniofacial skeleton. Although questionable, a wide intradural extension of the tumor should be considered.
Prospective, randomized controlled trials comparing survival outcomes of open versus purely endoscopic resections of ONB will likely never be done because of the rarity of this tumor as well as the need for a prolonged follow-up given its tendency for late recurrence2.
Adjuvant therapeutic modalities
Radiotherapy
Post-operative radiotherapy improves local control of the disease. However, for early stages (Kadish A or B)1 with negative resection margins, radiation is still questionable, and surgery alone might be enough. There is always a concern regarding the potential complications of the adjuvant radiation; however, with the advancement of radiation technologies, conformal techniques such as intensity-modulated radiotherapy and proton beam therapy have shown better outcomes improving local control and minimizing toxicity. There is no consensus regarding optimal dose delivery to the tumor bed.
Chemotherapy
Chemotherapy has a role only in advanced disease with distant metastatic tumors, recurrent tumor, or unresectable disease2. In the neoadjuvant setting, it decreases the size of the tumor, relief some compressive symptoms and helps in the complete surgical resection. It can be given in concomitance with radiation in an adjuvant setting for better results. The common drugs used are cisplatin, etoposide, adriamycin, vincristine, and cyclophosphamide22. The preferred chemotherapy regimen is cisplatin (33 mg/m2 daily) and etoposide (100 mg/m2 daily) for 3 days. Cisplatin is administered over 1 h in 250 mL normal saline after prehydration with 1 L normal saline over 1-2 h. Etoposide is given over 1 h in 250-mL 5% dextrose in water.
Management of the neck
Cervical lymphadenopathy at presentation is seen in 5-8% of patients2,22 and up to 30% of cases will have eventual involvement2. Patients with the advanced local disease should have a CT scan for neck metastases and regional treatment in the form of neck dissection and post-operative radiation therapy at the same time of management of the primary tumor in case of positive neck nodes22. In a retrospective analysis, Howell et al.23 described de pattern of regional ONB spread. Lymph node neck level II was most frequently involved in over 90% of cases, Levels I and III were involved in over 50% of cases and retropharyngeal nodes were positive in over 40% of cases. Levels IV and V were only involved in cases of widely disseminated neck metastases.
Distant metastases
In a systematic review and meta-analysis of 48 studies totaling 118 patients, Marinelli et al.24 found that, although rare, the most common location of distant metastatic disease was the bones (40%), drop spinal metastases (29%,) and lungs (29%). Chemotherapy in combination with surgery and/or radiation exhibited the best overall survival when compared to monotherapy and no treatment (p < 0.001). Platinum-based chemotherapy was most commonly utilized but did not provide a survival benefit when compared with all other regimens.
Prognosis and long-term follow-up
A meta-analysis of 26 studies (n = 390 patients) reported a mean overall survival at 5 years of 45% (SD 22), mean disease-free survival of 52% at 2 years (SD 21), 45% at 3 years (SD 23), and 41% at 5 years (SD 21). Few studies in the meta-analysis reported 10-year survival data, with an average of 52% (SD 27). Patients with metastases in cervical lymph nodes (on average 5% of the total) the survival was 29%, compared with 64% for patients with N0 disease (odds ratio 5.1). Survival according to the treatment modalities was 65% for surgery plus radiotherapy, 51% for radiotherapy and chemotherapy, 48% for surgery alone, 47% for surgery plus radiotherapy and chemotherapy, and 37% for radiotherapy alone14. Currently, the ideal modality, timing, and frequency of follow-up visits are not defined but should include clinical, endoscopic, and radiologic evaluation for at least 10 years given the ONB tendency for late recurrence. In our institution, we perform follow-up every 3 months during the 1st year, every 4 months for 3 years, then annually.
Conclusion
Currently, there are no formal guidelines outlining the treatment of ONB. There are four main factors that lead to the many opinions and controversies about diagnosis and management of ONB: (1) no individual clinician or institution will have more than a few cases a year; (2) ONB can easily be confused with other neoplasm of the nasal cavity; (3) the varying biological and clinical behavior ranging from relative indolent tumor to highly aggressive neoplasms with rapid, widespread metastases and tendency for late recurrence, and (4) the lack of large, multi-institutional, well-controlled prospective analyses limits meaningful conclusion regarding the best treatment modalities. Surgery, followed by radiotherapy, and chemotherapy for metastatic and recurrent disease, remain the mainstay of the treatment for ONB. Transdisciplinary research of ONB at the genetic and molecular level is needed.











 nova página do texto(beta)
nova página do texto(beta)



