Introduction
The gastrointestinal tract is an important immune organ of the human body and is colonized by a huge population of microorganisms, both nonpathogenic (probiotics) and pathogenic, consisting of 400-500 different species at about 1011 cfu per gram of intestinal tissue1,2. Intestinal microflora is defined as the population of live microorganisms in the gastrointestinal tract, which acts as a separate ecosystem and affects patients' health and longevity3,4.
The balance of the intestinal flora can be adjusted with pharmacological or dietary factors, such as the administration of probiotics and prebiotics. Probiotics, as living organisms, when taken in adequate quantities can especially affect the intestine microflora, reduce bacterial translocation and contribute to the restoration of the intestinal epithelial barrier and the maintenance of the functional integrity of the intestinal mucosa3-8. Prebiotics are non-degradable food ingredients that have a beneficial effect on the host by eclectically stimulating the proliferation and/or the activity of one single bacteria or a group of bacteria of the large intestine9. They contribute to the diversity of the intestinal microflora, increasing the number of protective intestinal bacteria and the production of short chain fatty acids in the colon and decreasing the production of cytokines that promote inflammation6-8. Synbiotics, which are defined as the combination of probiotics and prebiotics, seem to protect against inflammation10.
Ulcerative colitis (UC) is a chronic disease of the gastrointestinal tract, especially the colon that follows an unpredictable course characterized by multiple episodes of flairs and remissions. Several factors, such as genetic, environmental and bacterial, seem to be involved in the pathogenesis of UC11,12. Many experimental models have been used to study the pathophysiology and therapy of this inflammatory bowel disease13. In rodents, the supplementation of drinking water with dextran sulfate sodium (DSS) can induce an experimental model of UC12-14. Per os administration of 5% DSS in the drinking water of BALB/c mice caused acute and chronic colitis with several histopathological similarities to UC15; thus, a replicable model of UC in Swiss Webster rats has been developed16,17. Furthermore, the clinical, structural, and pathological findings of the experimental colitis with DSS in Wistar and Sprague-Dawley rats are well described18. The aim of the current study was to estimate the effects of synbiotics on the clinical, laboratory, macroscopic, and histopathologic features in the Wistar rat model of DSS-induced experimental colitis.
Materials and methods
Animals
A protocol was designed to minimize discomfort and pain in the animals. For the current experimental study, 40 male pathogen-free Wistar rats, 2.5 months old and weighing 250-300 g, were used. All animals during the study were housed in cages of one to four positions, depending on the experiment period, under normal laboratory conditions with temperature between 18°C and 22°C, relative humidity between 55 and 65% and a land-controlled light cycle of 12 h light and 12 h dark. The animals had free access to water and food (ad libitum), which was special for rats. The genetic standardization of animal models was a randomized series of the experimental animals and the microbial standardization was normal (conventional) experimental animals. The experiments took place at the physiology laboratory of the Veterinary School of Aristotle University of Thessaloniki after authorization and approval by the Veterinary Service of Thessaloniki (Number of experimentation approval 13/1144/24-1-08), in accordance with the requirements of the EU Directive 86/609/EEC about protecting animals that are used for experimental or other scientific purposes.
Induction of UC with DSS 5%
Rats received 5% dextran sodium sulfate-DSS (molecular weight: 36 kDa ± 50 kDa; MP Biomedicals Inc., Cleveland, OH, USA) in their drinking water for 8 days until the presence of loose stools, diarrhea, and macroscopic rectal bleeding. After 8 days, DSS was stopped, and eight rats were sacrificed to confirm the presence of the experimental UC.
Groups
Rats were randomly divided into three groups: DSS, CONTROL, and SYNBIOTICS.
DSS group (n = 8): after 8 days of DSS administration, the rats were sacrificed to confirm the presence of experimental UC and study the histopathologic features of the colon. The colonic tissue was examined macroscopically and microscopically to determine the presence of experimental UC and establish baseline measurements to compare the experimental findings.
CONTROL group (n = 16): after the DSS administration had been stopped, these rats received tap water and no other medication. Subgroup CONTROL 4 (n = 8): rats were sacrificed on the 4th day post-DSS. Subgroup CONTROL8 (n = 8): rats were sacrificed on the 8th day post-DSS.
SYNBIOTICS group (n = 16): after the DSS administration had been stopped, these rats received a synbiotic compound (Ecologic® 825-Winclove Bio Industries Amsterdam, The Netherlands) once per day through an orogastric catheter. The synbiotic regimen used was a mixture of probiotics and prebiotics in the form of powder. The active ingredients were: prebiotics, including Cornstarch, maltodextrin, fructo-oligosaccharides, inulin, minerals (potassium chloride, magnesium sulfate, and manganese sulfate), enzymes (amylases; and contained soy), and vanilla; and nine probiotic strains (contained soy and milk components), including Bifidobacterium bifidum (W23), Bifidobacterium lactis (W51), B. lactis (W52), Lactobacillus acidophilus (W22), Lactobacillus casei (W56), Lactobacillus paracasei (W20), Lactobacillus plantarum (W62), Lactobacillus salivarius (W24), and Lactobacillus lactis (W19) with 2.5 × 109 colony-forming units (CFU) per gram. The administrated dose of probiotics was 2 × 108 CFU per rat per day. Subgroup SYNBIOTICS 4 (n = 8): rats were sacrificed on the 4th day post-DSS. Subgroup SYNBIOTICS 8 (n = 8): rats were sacrificed on the 8th day post-DSS.
Euthanasia
Euthanasia was carried out by cardiac puncture and exsanguination, which was performed under deep anesthesia with the intraperitoneal administration of chloral hydrate (400 mg/kg body weight).
Sample recovery
Median laparotomy was performed after shaving the abdomen and using 10% Povidone-iodine solution for skin antisepsis. Under aseptic conditions, a 4-cm midline ventral abdominal incision was made from the height of the xiphoid, allowing entry into the peritoneal cavity, and exposure of the large intestine. The large intestine was mobilized from the ileocecal valve to the anal canal and excised. The distal colon was first opened along the anti-mesenteric border and washed with saline, then was weighed, measured, and photographed and the macroscopic intestinal mucosal lesions were recorded.
Next, the entire large intestine was fixed in neutral formalin for histopathological examination. Furthermore, in every rat, a cardiac puncture was performed to retrieve sufficient amounts of blood for the laboratory examinations. The blood samples were placed in veterinary vials IDEXX VetCollect 1.0 ml with anticoagulant K3 EDTA. A sterile syringe with a needle of 26G diameter was used to obtain 5 ml of blood from each rat.
Body weight and laboratory assessment of colonic inflammation
Body weights were recorded daily. Hemoglobin (HB) concentration, hematocrit (HT), total erythrocyte numbers (red blood cell [RBC]), mean corpuscular volume (MCV), mean corpuscular HB (MCH), and platelets (PLT) count were determined. The blood tests were performed by the veterinary analyzer Scil Vet abc (Animal Blood Counter) with a special program for rats.
Histology, immunohistochemistry, and morphometry
Tissues were fixed in 10% neutral formalin solution and embedded in paraffin. Histopathology sections were cut at a thickness of 4-6 µm and stained with hematoxylin and eosin (HE). Immunohistochemical staining was used for myeloperoxidase (MPO). 5-µm thick formalin-fixed, paraffin-embedded tissue sections were used. The primary antibodies used for MPO immunohistochemistry included rabbit anti-MPO (MPO Ab-1; Thermo Fischer Scientific Lab Vision, Fremont, CA, USA). Heat-induced antigen retrieval was performed with citrate buffer, pH 6, for MPO. Primary antibody binding was detected with goat anti-rabbit IgG polymerized horseradish peroxidase (Chemicon International, Temecula, CA, USA). The signal was detected with diaminobenzidine and tissues were counterstained with hematoxylin12.
Colitis was graded on a scale of 0-4 with ascending severity (Grade 0: no lesions; Grade 1: minimal lesions; Grade 2: mild lesions; Grade 3: moderate lesions; and Grade 4: severe lesions), which was first used by Berg et al.19, but was modified later20.
Neutrophil infiltration was quantified using morphometry by assessment of MPO-stained slides of colon tissue sections. In short, using a technique described elsewhere12,21, multiple representative high-power fields were captured with a Nikon DS-5M-L1 digital camera (Nikon, Tokyo, Japan) (× 40). Ten images per treatment group were randomly selected. Then, the identified neutrophils in each image were counted using the cell count plug-in of the ImageJ image processing and analysis program (NIH, Bethesda, MD, USA).
Statistical analysis
All data were reported as mean ± standard deviation. Statistical analysis for colon length and blood tests, which presented normal distributions, was performed using deviation analysis (Analysis of variance [ANOVA]) and the comparison of means was made using the Bonferroni correction for post hoc pairwise comparisons. Body weights were analyzed with repeated measures ANOVA. Colitis score and MPO, which did not have normal distribution, were analyzed using the non-parametric Kruskal-Wallis test. Statistical analysis was performed using DOE Prisma© Software (GraphPad Software Inc., San Diego CA, USA). p < 0.05 was considered statistically significant.
Results
Colitis presentation
On the 8th day of DSS treatment, all animals developed severe colitis with diarrhea and hematochezia.
Body weight
After the first 8 days of DSS administration, body weights decreased in both groups, but the differences were not significant. After 8 days of treatment, the body weight increased in all groups, but the differences were not statistically significant. Figure 1A depicts the results.

Figure 1 A: body weight comparison at the beginning, middle and end of the experiment (mean). No statistically significant difference was noted. B: distal colon length comparison in the three groups. Statistically significant difference between the SYNBIOTICS 4 and CONTROL 4 groups. C: hemoglobin comparison of the three groups. No statistically significant difference was noted. D: hematocrit comparison in the three groups. Statistically significant difference between the SYNBIOTICS 8 and CONTROL 8 groups. E: red blood cell count comparison in the three groups. No statistical significant difference was noted. F: mean corpuscular volume comparison in the three groups. Statistically significant difference between the SYNBIOTICS 8 and CONTROL 8 groups. G: mean corpuscular hemoglobin comparison in the three groups. Statistically significant difference between the SYNBIOTICS 4 and CONTROL 4 groups. H: platelet comparison in the three groups. No statistically significant difference was noted.
Distal colon length
The mean colon length differed between the groups (p < 0.0001). The mean colon length increased in both the CONTROL and SYNBIOTICS groups, both in the 4th and 8th post-DSS days compared to the DSS group. The increase was not statistically significant for the control group compared to the DSS on the 4th day, but was significant on the 8th day (p = 0.0301), while the increase was significant for the SYNBIOTICS group in both the 4th and 8th days post-DSS (p = 0.0192 and p = 0.004, respectively). The mean colon length in the SYNBIOTICS group was significantly higher than the CONTROL group on the 4th day (p = 0.0241), but not on the 8th day post-DSS. The increase of the colon length from the 4th to the 8th post-DSS day was statistically significant for the CONTROL group (p = 0.0375), but not for the SYNBIOTICS group. Figure 1B depicts the results.
Hematological variables
The disease severity and the number of hemorrhagic bowel movements affected the values of HB, HT, erythrocytes, MCV, MCH, and PLT. The hematological variables, except for erythrocytes (RBCs) (p = 0.96) and PLTs (p = 0.40), differed significantly between the groups. Specifically, statistically significant differences were found for HB (p < 0.001), HT (p < 0.001), MCV (p = 0.0016), and MCH (p = 0.004). While HB and HT increased on the 4th day post-DSS in both groups compared to the DSS group, the increase was significant only on the 8th day post-DSS in both the CONTROL (p = 0.0001 and p = 0.0011) and SYNBIOTICS groups (p < 0.001 and p < 0.001), respectively. Furthremore, the increases in HB and HT were significant from the 4th to the 8th day post-DSS, both in the CONTROL group (p = 0.0003 and p = 0.0318) and SYNBIOTICS group (p < 0.0001 and p < 0.0001). Furthermore, the HT was significantly higher in the SYNBIOTICS group on the 8th post-DSS day compared to the CONTROL group (p = 0.0318). Regarding MCV and MCH, there were also statistically significant differences between the groups (p = 0.0016 and p = 0.0040, respectively). Specifically, both MCV and MCH decreased significantly in the SYNBIOTICS 4 group compared to DSS (p = 0.0453 and p = 0.0188, respectively). Moreover, MCV increased on the 8th day post-DSS in the CONTROL group compared to the 4th day (p = 0.0224) and compared to the SYNBIOTICS group on the 8th day (p = 0.0453), while MCH increased in the CONTROL group on the 4th post-DSS day compared to the SYNBIOTICS group. Figure 1C-H depicts these results.
Macroscopic and histopathological findings
After the administration of DSS for 8 days, experimental severe colitis was histopathologically confirmed. Specifically, in the descending colon and in the rectum, multifocal, and diffuse erosions of the intestinal mucosa and enlargement of the lumen of the glands with simultaneous aggregation of a large number of inflammatory cells (mainly neutrophils, lymphocytes, plasma cells and macrophages and, secondarily, eosinophils) were observed (Fig. 2A). Focally and at a more advanced stage, colitis was characterized by a loss of glands, the development of connective tissue and reduction of the mucosa thickness. Furthermore, dysplasia of the glands was noted, with the dysplastic glands being characterized by a helical shape and significant enlargement of their lumen. Dysplastic epithelium displayed a loss of goblet cells, loss of cell nucleus polarity, and cell pleomorphism.
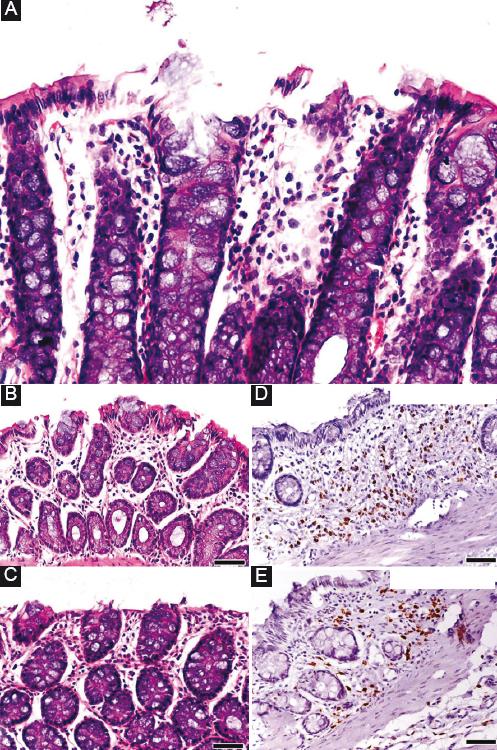
Figure 2 A: intestinal mucosa of the descending colon after 8 days of dextran sulfate sodium (DSS) administration. Erosion of the epithelium and mild increase of the inflammatory cells were observed. B and C: after 8 days of treatment, there was no difference in the histopathological findings between the CONTROL group and the SYNBIOTICS group. D and E: however, regarding the number of myeloperoxidase + cells (neutrophils), there was a significant decrease in the SYNBIOTICS group compared to the CONTROL.
While synbiotics administration slightly decreased the colitis score and led to mild improvement of intestinal mucosal erosions compared to the CONTROL group on both the 4th and 8th post-DSS days, these differences were not significant (Fig. 2B and C). Figure 3A and B depicts these results.
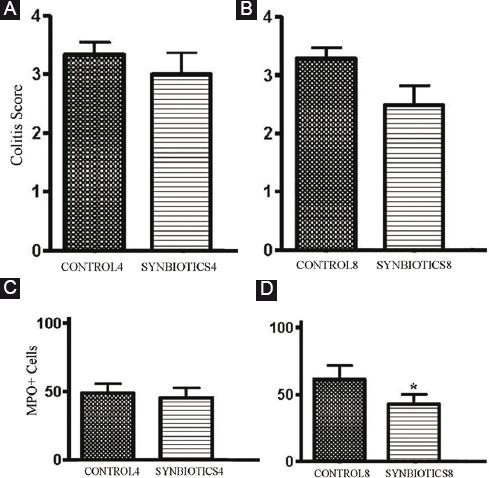
Figure 3 A: colitis score comparison between the SYNBIOTICS and CONTROL groups on the 4th day post-dextran sulfate sodium (DSS). B: colitis score comparison between the SYNBIOTICS and CONTROL groups on the 8th day post-DSS. C: myeloperoxidase (MPO) + cells comparison between the SYNBIOTICS and CONTROL groups on the 4th day post-DSS. D: MPO + cells comparison between the SYNBIOTICS and CONTROL groups on the 8th day post-DSS. Statistically significant difference between the SYNBIOTICS 8 and CONTROL 8 g.
MPO activity
MPO-specific immunohistochemical staining analysis revealed a reduction of MPO positive cells (mainly neutrophils) in the SYNBIOTICS group compared to the control group, but was statistically significant only on the 8th day post-DSS (p < 0.001) (Fig. 2D and E). Figure 3C and D depicts these results.
Discussion
The development of UC is attributed to the activation of an immune response in a subset of non-pathogenic colonic bacteria in genetically-predisposed individuals. Clinical and experimental studies have shown that the relative balance between pathogenic and protective bacteria of the intestinal microflora changes in UC22. The main pharmaceutical therapy for UC includes the use of anti-inflammatories, immunosuppressants, and antibiotics. Dietary and nutritional factors are required in all types of inflammatory bowel disease, but this is often overlooked. The role of nutrition is not only significant because it achieves maintenance of the disease in remission but also because it helps in the reduction of its activity. The intestinal microflora is in a synbiotic relationship with eukaryotic mucosal cells and includes approximately 1014 bacteria comprising more than 400 different species23,24. The necessity of dietary factors in relation to the pathogenesis and therapy of UC has been highlighted in recent years. Nutritional factors can modulate the intestinal immune response, improve the function of the intestinal mucosal barrier, and modify the intestinal flora. Synbiotics (probiotics and prebiotics) present beneficial effects by improving the balance of the gut microbial flora. The use of prebiotics contributes to the increase of intestinal mucous, the maintenance of its functional integrity, the balance of body water and electrolytes, the supply of the body with energy and dietary factors, the increase of resistance to pathogenic microorganisms, the adjustment of the intestinal mucous immune response, and the stimulation of the humoral immune response. Furthermore, prebiotics seem to play a significant role in the maintenance of the intestinal flora or the restoration of the disturbed equilibrium of the intestinal flora. This is achieved by the enhancement of intestinal barrier function, which results in the inhibition of bacterial translocation25,26. The most known prebiotics are inulin and oligofructose, carbohydrates that are natural ingredients found in plants7,27. Probiotics play a main role in the formulation of the large intestine's natural ecosystem. The most known and widely used probiotic microorganisms belong to the genus Lactobacillus and Bifidobacterium28-30. Probiotics contribute to the maintenance of anatomical and functional integrity of the intestinal mucosa. The administration of probiotics contributes to the improvement and increase of the intestinal epithelial barrier by suspending the epithelial cell apoptosis. Probiotics inhibit the relocation of pathogenic microorganisms, such as Escherichia coli, and exclude pathogenic microorganisms by occupying the available natural space for intestine colonization. Furthermore, they excrete antimicrobial substances that destroy pathogenic microorganisms before they can colonize the intestine23.
The structural changes of the intestinal mucous caused by the administration of DSS allow lumen antigens to penetrate, resulting in increased neutrophil infiltration and an increase of MPO in the intestinal mucous, which triggers an inflammatory reaction. Furthermore, DSS promotes the production of chemokines from the epithelial cells of the intestinal mucous, resulting in a higher concentration of neutrophils. Histopathologically, the experimental models share almost all the morphological characteristics and a similar allocation of lesions with the UC affecting humans. Per os DSS administration lead to the development in all animals of severe colitis with diarrhea and hematochezia and characteristic histopathological findings, as we have already described elsewhere12,14.
In the current study, with regard to the hematological variables, synbiotics administration lead to significantly increased HT on the 8th post-DSS day compared to control. Many studies have focused on the efficiency of probiotics administration for colitis and included them in UC treatment31-37. Recent clinical studies have found that probiotics can play a significant role in the treatment of UC. In one of these, Venturi et al.38 studied the actions of a probiotic regimen from several species (three chains bifidobacteria, four chains lactobacilli, and one chain of Streptococcus salivarius sp. Thermophilus) for 12 months in 20 patients with inactive UC and with intolerance to mesalazine. The results showed that 15 of 20 patients remained under remission. Venturi's study is considered unique in investigating probiotics' action in the stools' flora, noting an increase of bifidobacteria, lactobacilli, and S. salivarius without a change in other species. In another double blind study, the probiotic drug E. coli Nissle 1917 showed efficacy and safety in maintaining remission equivalent to the gold-standard mesalazine in patients with UC39. In another study, the VSL#3 probiotic administration to patients with mild-to-moderate UC that did not respond to conventional therapy resulted in a combined induction of remission/response rate of 77%, with no adverse events40. Furrie et al.41 found similar results in a double-blind clinical study comparing synbiotics with a placebo. The administration of synbiotics to 18 patients caused a significant reduction of inflammatory cytokines (tumor necrosis factor [TNF]-a, and interleukin [IL]-1b) and the histopathological examination revealed a reduction of inflammatory cell infiltration.
Furthermore, synbiotics administration ameliorated epithelial erosion and resulted in a lower colitis injury score and reduction of the intestinal mucosal inflammation, although the differences were not statistically significant. However, increases in colon length were significant for the synbiotics administration compared to the control on the 4th day, but not the 8th. Many experimental studies have shown that the administration of prebiotics, probiotics, and synbiotics can be effective for UC in many ways. Videla et al.42 estimated the effectiveness of administering inulin, a prebiotic, for experimental colitis. Experimental colitis was caused by the administration of 5% DSS in rats. The administration of inulin improved the histopathological findings, reduced the release of inflammatory mediators, and reduced the concentration of MPO. In this case, the effect seemed to be mediated by the modification of the intracolonic milieu. Moreover, it has been shown that prior administration of probiotic bacteria reduces mucosal inflammation and damage in DSS-induced colitis. One particular study showed that DSS colitis was associated with significant changes in the fecal anaerobic bacterial flora and these changes were modulated by the administration of probiotic bacteria43. Likewise, it has been reported that the administration of synbiotic combinations improves DSS-induced acute colitis and has an anti-inflammatory effect, resulting in improvement in the full clinical appearance of chronic inflammation and the reduction of colonic IL-1a, MPO activity, TNFa, and IL-1a42.
In our experimental study, the administration of synbiotics 8 days after the administration of DSS caused a statistically significant reduction of the quantity of MPO-positive cells (mainly neutrophils) compared to control. Osman et al.44 observed that the daily administration of synbiotics (Bifidobacterium infantis, oligofructose, and inulin) reduced inflammatory reactions in the experimental DSS colitis in rats, specifically in the MPO activity of the intestinal mucosa of the large bowel, compared to control. Furthermore, there was a statistically significant reduction in bacterial relocation, both at the mesenteric lymph nodes and at the liver lobe in the group that received synbiotics. Fukuda et al.45 compared the therapeutic results of the administration of prebiotics (glutamine-rich protein and hemicellulose-rich dietary fiber), probiotics (Lactobacillus and Clostridium butyricum), or antibiotics (vancomycin and metronidazole) in the experimental DSS-colitis in rats. They observed that treatment with the administration of prebiotics significantly reduced mucosal inflammation in contrast with the administration of probiotics or antibiotics. There is an indication that probiotics stimulate the expression of the MUC3 gene that produces mucin, a glycoprotein that is excreted from the epithelial cells in the gastrointestinal system. The expression of mucin increases the protection of the gastrointestinal tract from viruses and microbes46. Furthermore, at an experimental level, L. plantarum and Lactobacillus rhamnosus GG, and the synthetic probiotic mixture VSL#3, can increase the production of IL-10 and the species lactobacilli and bifidobacteria can reduce the production of TNF-a and Interferon (INF)-γ29,47,48. Furthermore, it has been demonstrated that probiotics, due to their protective immunomodulatory capacity, prevents the growth of spontaneous colitis in rats that present a deficiency of IL-10 and also reduce the excretion of the pro-inflammatory cytokines TNF-a and INF-γ from the intestinal mucosa49,50. The administration of the synthetic probiotic mixture VSL#3 (includes 4 Lactobacillus sp., 3 Bifidobacterium spp. and S. salivarius sub spp. thermophilus) seems to restore the function of the intestinal barrier in chronic experimental colitis7.
Both prebiotics and probiotics have specific actions by which they act and ameliorate UC, and this action in case of synbiotics is enhanced, as synbiotics, as a combination of prebiotics and probiotics, are more effective against UC than prebiotics or probiotics alone. The exact mechanism of actions of synbiotic administration in UC is not yet fully elucidated but it is seems that the synergy between prebiotics and probiotics, rather than the specific action of its one, leads to a modulation of the inflammatory response reducing the production of pro-inflammatory cytokines, increasing the expression of anti-inflammatory cytokines, modulating the immune cell function, and stabilizing the intestinal mucosal barrier51.
Conclusions
The administration of 5% DSS to Wistar rats produces variably severe symptoms and significant structural and ultrastructural alterations similar to human UC, including epithelial disruption, focal lesions, and superficial inflammation. Collectively, the results of our study suggest a beneficial role of synbiotics in the experimental DSS-induced UC, as animals fed a synbiotics-rich diet exhibited a significant increase in HT and colon length and a trend toward histopathological and clinical improvement, which were associated with a significant decrease in the MPO positive cells that are normally present in colitis and in neutrophil infiltration. Regarding the possible mechanisms of action, prebiotics contribute to the improvement of the epithelial intestinal barrier, to the maintenance of the functional integrity of the intestinal mucous, to the increase of resistance against pathogenic microorganisms, to the adjustment of the intestinal mucosa immune response, and to the stimulation of the humoral immune response. Probiotics initially eliminate pathogenic microorganisms by occupying the available physical space for colonization in the intestine and secrete antimicrobial substances that destroy pathogenic microorganisms before they colonize the intestine. Moreover, given the fact that they do not induce significant side effects, synbiotics could be included in human UC treatment.











 nueva página del texto (beta)
nueva página del texto (beta)


