Introduction
Acute appendicitis is one of the most common surgical pathologies, affecting 7-9% of the general population. This pathology does not have gender or age preference but is more frequent in the 2nd and 3rd decades of life. The therapeutic approach preferred is surgery, with a tendency for the laparoscopic approach1. Complications are associated with the time since the start of symptoms up to surgical intervention. In the case of surgical site infections, these are classified according to their depth in superficial or organ space, latter with mayor complexity2. Complicated acute appendicitis (CAA) is considered type III (gangrenous) and type IV (perforated). Pathology's mortality rate fluctuates between 1.5% and 10.8%3. There are different regimes indicated as post-operative antibiotic treatment, but ciprofloxacin/metronidazole is considered as the standard of care. However, carbapenems are also recommended as empiric therapy, due to its bactericidal action over different bacteria present in the intestinal flora4,5.
The Hospital Teodoro Maldonado Carbo (HTMC) is the largest public hospital along with the Ecuadorian coast, serving an essential segment of the country's population. As a center of reference, more than 50% of acute appendicitis treated are complicated. In this hospital, we observed a favorable response to carbapenems (ertapenem, meropenem, or imipenem) in terms of early therapeutic action, less number of complications, and hospitalization. The study aims to prove the efficacy of carbapenems versus ciprofloxacin/metronidazole in CAA post-operative evolution, in post-operative complication prevention, and reduction of hospital stay. As a secondary objective, it was proposed to verify the efficacy between the different carbapenems (ertapenem, meropenem or imipenem) for the objective mentioned previously, through a subanalysis.
Materials and methods
Study design
This is an observational, analytic, longitudinal, and prospective cohort study. It was carried out in patients with surgical approach due to CAA in HTMC (Guayaquil, Ecuador) from March 2014 to November 2016. The present study was presented in accordance with the STROBE statement.
Population selection
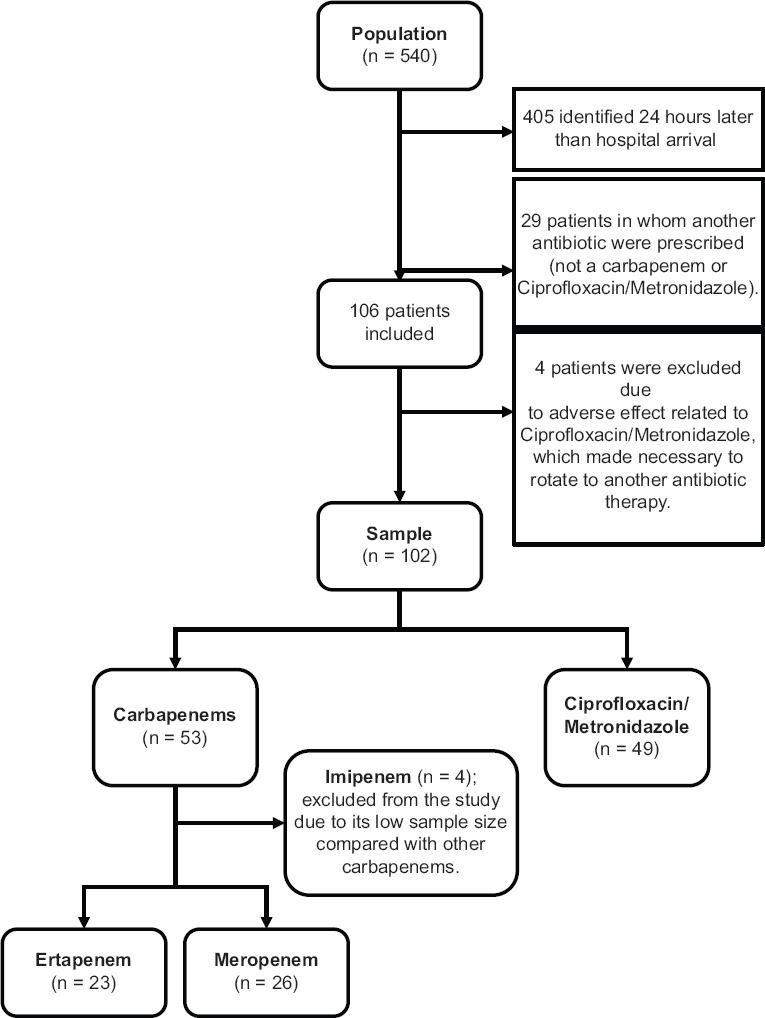
The universe of the study included patients treated by the emergency department of the HTMC due to CAA during 2015. The inclusion criteria were patients ≥ 15 years that assisted at HTMC by their own means or referrals of primary or secondary health-care units, patients in whom clinical follow-up in the first 24 h after their arrival were possible, and patients that received any carbapenem (ertapenem, meropenem, or imipenem) or ciprofloxacin/metronidazole. For each patient that received ciprofloxacin/metronidazole, a patient that received carbapenem was included in the study. The exclusion criteria were patients that received any antibiotic treatment in the past 15 days, history of antibiotic allergies, creatinine clearance ≤ 40 ml/min, and history of hepatic insufficiency, neutropenia, gestating period, or lactation.
Follow-up and data collection
The following parameters evaluated antibiotic response: (a) clinical status (cured, stable, and worsened), presence of intra-abdominal or surgical site infection, and antibiotics' side effects; (b) antibiotic therapy rotation and white blood cell (WBC) count; and (c) hospital stay. The first parameter was documented in three different post-operative times, independently from hospital discharge: immediate (first 72 h after surgery), mediate (until 14 days after surgery), and late (15-30 days after surgery). A data collection form was designed to obtain data related to the study. Collected data were then tabulated in an electronic collection form.
Statistical analysis
We considered a type I error (alpha) of 5%, type II error (beta) of 20%, and therapeutic response between meropenem and clindamycin/tobramycin for the management of intra-abdominal infections of surgical resolutions described by Wilson (91% vs. 86%, p > 0.05)6. The sample size was estimated with the non-inferiority and equivalence test7, with a statistical power of 80%. Quantitative variables were described by mean (standard deviation) or median (minimum-maximum range), taking on account statistical distribution (Kolmogórov-Smirnov or Shapiro-Wilk test). Qualitative variables were described by frequency (%). Quantitative variable contrast was done with the Student's t-test and Welch or Mann-Whitney U-test, and qualitative variable contrast was done through Pearson's Chi-squared test or Fischer's exact test. The association between antibiotic therapy and antibiotic rotation necessity was estimated through relative risk (RR). Difference between antibiotic length and hospital stay between the study groups was contrasted with Gray's test and described with a cumulative curve. A subanalysis between carbapenems was made. p < 0.05 was considered statistically significant. Data analysis was performed with R v3.6.0 (R Foundation for Statistical Computing; Vienna, Austria).
Ethical aspects
The presented study respected the stipulations of the Declaration of Helsinki (2008). Approval of the Medical Faculty Graduate School Institutional Review Board of Universidad Espíritu Santo (UEES) and Clinical Research and Teaching Department of HTMC was obtained. All patients included in the study signed informed consent.
Results
It was estimated that each group and subgroup should have 23 subjects approximately. A total of 102 subjects were consecutively selected and followed during the study period: 53/102 (51.9%) received carbapenem (23/53 ertapenem, 26/53 meropenem, and 4/53 imipenem); meanwhile, 49/102 (48.1%) received ciprofloxacin/metronidazole. Thereafter, patients treated with imipenem were excluded from the study due to its low sample size compared with others carbapenems (Fig. 1).
The median age was 33 years, 33.7% of cases were feminine. Approximately 9% of the study population had a clinical history of diabetes mellitus and hypertension, among others. There were 12/98 (12.2%) cases with signs of sepsis and 76/98 (77.6%) cases which corresponded to acute perforated appendicitis. In 61/98 (62.2%) cases, the surgical procedure was performed within 24 h after the subjects' admission at HTMC. In 82/98 (83.7%) patients, a laparotomic approach was performed. Surgical duration's median was 2 h. Table 1 summarizes sociodemographic characteristics among antibiotic therapies.
Table 1 Baseline characteristics of the study population
| Baseline characteristics | Total (n = 98) | Carbapenems (n = 49) | Ciprofloxacin/metronidazole (n = 49) | p-value |
|---|---|---|---|---|
| Age(years), median(range) | 33(15-92) | 33(15-92) | 33(15-87) | 0,577a |
| Young adults(< 40) | 63(64.3) | 33(67,3) | 30(61,2) | |
| Adults(40-65) | 23(23,5) | 12(24,5) | 11(22,4) | |
| Elderly(≥ 65) | 12(12,2) | 4(8,2) | 8(16,3) | |
| Gender, n(%) | 0,087b | |||
| Feminine | 33(33,7) | 21(42,9) | 12(24,5) | |
| Masculine | 65(66,3) | 28(57,1) | 37(75,5) | |
| Clinical history, n(%) | ||||
| Diabetes mellitus | 9/102(9,2) | 5/49(10,2) | 4/49(8,2) | 1,000b |
| Hypertension | 9/102(9,2) | 4/49(8,2) | 5/49(10,2) | 1,000b |
| Others | 9/102(9,2) | 5/49(10,2) | 4/49(8,2) | 1,000b |
| Presenting symptoms, n(%) | 0,355b | |||
| Sepsis | 12(12,2) | 4(8,2) | 8(16,3) | |
| SIRS | 86(87,8) | 45(91,8) | 41(83,7) | |
| Type of appendicitis, n(%) | 1,000b | |||
| Gangrenous | 22(22,4) | 11(22,4) | 11(22,4) | |
| Perforated | 76(77,6) | 38(77,6) | 38(77,6) | |
| Intervention delay, n(%) | 0,677b | |||
| < 24 h | 61(62,2) | 29(59,2) | 32(65,3) | |
| ≥ 24 h | 37(37,8) | 20(40,8) | 17(34,7) | |
| Creatinine clearance(ml/min), median(range) | 74,1(40,2-192,0) | 75,9(40,9-192,0) | 72,4(40,2-192,0) | 0,316a |
| ≥ 90 ml/min | 35(35,7) | 16(32,7) | 19(38,8) | |
| 60-89 ml/min | 41(41,8) | 21(42,9) | 20(40,8) | |
| 40-60 ml/min | 22(22,4) | 12(24,5) | 10(20,4) | |
| Type of surgery, n(%) | 0,239b | |||
| Laparoscopic | 16(16,3) | 10(20,4) | 6(12,2) | |
| Laparotomic | 29(29,6) | 11(22,4) | 18(36,7) | |
| McBurney | 53(54,1) | 28(57,1) | 25(51,0) | |
| Surgical procedure time(hh:mm), median(range) | 02:00(01:00-03:20) | 02:00(01:00-03:20) | 02:00(01:00-03:00) | 0,642a |
aMann-Whitney U-test;
bPearson's Chi-squared test
In contrast with ciprofloxacin/metronidazole, carbapenem cases were associated with a significant lower appearance of intra-abdominal or surgical site infection through immediate (2/49 vs. 12/49; p = 0.014), mediate (3/49 vs. 27/49; p < 0.001), or late (1/49 vs. 10/49; p = 0.010) follow-up. Furthermore, a significant higher frequency of cured cases managed with carbapenems was identified though mediate (35/49 vs. 15/49; p < 0.001) and late (45/49 vs. 32/49; p < 0.001) follow-up. There was not any significant difference in clinical outcomes between carbapenems (ertapenem or meropenem) during the post-operative time (Table 2).
Table 2 Therapeutic results according to the antibiotic scheme
| Therapeutic results | Carbapenems (n = 53) | Ciprofloxacin/ metronidazole (n = 49) | p-value* | Ertapenem (n = 23) | Meropenem (n = 26) | p-value* |
|---|---|---|---|---|---|---|
| Immediate follow-up (first 72 h after surgery) | ||||||
| Clinical status | 0,264 | 0,397 | ||||
| Cured | 2(4,1) | 0(0,0) | 1(4,3) | 1(3,8) | ||
| Stable | 45(91,8) | 45(91,8) | 22(95,7) | 23(88,5) | ||
| Worsened | 2(4,1) | 4(8,2) | 0 | 2(7,7) | ||
| Infection | 0,014 | 0,398 | ||||
| Intra-abdominal | 1(2,0) | 3(6,1) | 0 | 1(3,8) | ||
| Surgical site | 1(2,0) | 9(18,4) | 0 | 1(3,8) | ||
| None | 47(95,9) | 37(75,5) | 23(100,0) | 24(92,3) | ||
| Adverse effect | 0,241 | 0,913 | ||||
| Headache | 0 | 0 | 0 | 0 | ||
| Diarrhea | 3(6,1) | 0 | 2(8,7) | 1(3,8) | ||
| None | 46(93,9) | 49(100,0) | 21(91,3) | 25(96,2) | ||
| Mediate follow-up (until 14 days after surgery) | ||||||
| Clinical status | < 0,001 | 0,561 | ||||
| Cured | 35(71,4) | 15(30,6) | 16(69,6) | 19(73,1) | ||
| Stable | 13(26,5) | 25(51,0) | 6(26,1) | 5(19,2) | ||
| Worsened | 1(2,0) | 9(18,4) | 1(4,3) | 0 | ||
| Infection | < 0,001 | 0,558 | ||||
| Intra-abdominal | 1(2,0) | 6(12,2) | 1(4,3) | 0 | ||
| Surgical site | 2(4,1) | 21(42,9) | 1(4,3) | 1(3,8) | ||
| None | 46(93,9) | 22(44,9) | 21(91,3) | 25(96,2) | ||
| Adverse effect | 0,368 | 0,951 | ||||
| Headache | 0 | 1(2,0) | 0 | 0 | ||
| Diarrhea | 1(2,0) | 0 | 1(4,3) | 0 | ||
| None | 48(98,0) | 48(98,0) | 22(95,7) | 26(100,0) | ||
| Late follow-up (15-30 days after surgery) | ||||||
| Clinical status | < 0,001 | 0,397 | ||||
| Cured | 45(91,8) | 32(65,3) | 22(95,7) | 23(88,5) | ||
| Stable | 2(4,1) | 17(34,7) | 1(4,3) | 1(3,8) | ||
| Worsened | 2(4,1) | 0 | 0 | 2(7,7) | ||
| Infection | 0,010 | 0,951 | ||||
| Intra-abdominal | 0 | 0 | 0 | 0 | ||
| Surgical site | 1(2,0) | 10(20,4) | 1(4,3) | 0 | ||
| None | 48(98,0) | 39(79,6) | 22(95,7) | 26(100,0) | ||
| Adverse effect | - | - | ||||
| Headache | 0 | 0 | 0 | 0 | ||
| Diarrhea | 0 | 0 | 0 | 0 | ||
| None | 49(100,0) | 49(100,0) | 23(100,0) | 26(100,0) | ||
*Pearson's Chi-squared test
Imipenem was excluded from carbapenems' subanalysis because there were fewer cases than estimated in the sample size calculation.
The antibiotic therapy rotation was needed in 7/98 (7.1%) cases, all associated with ciprofloxacin/metronidazole treatment (7/49, 14.3%; p = 0.006). Carbapenems permitted to reduce antibiotic rotation necessity (RR = 0.462; 95% confidence interval 0.369-0.576; p = 0.012). The mean antibiotic therapy length until rotation of subjects who received carbapenem was 3.44 days (range 1-14); meanwhile, in subjects with ciprofloxacin/metronidazole was 4.14 days (range 1-14) (p = 0.012) (Fig. 2). There was a statistically significant difference in the median WBC between those who used carbapenems versus ciprofloxacin/metronidazole by the 5th day of the antibiotic regime (10.000 vs. 14.000 WBC per high-power field, respectively; p = 0,043), but any other difference was seen in corresponding subanalysis between carbapenems.
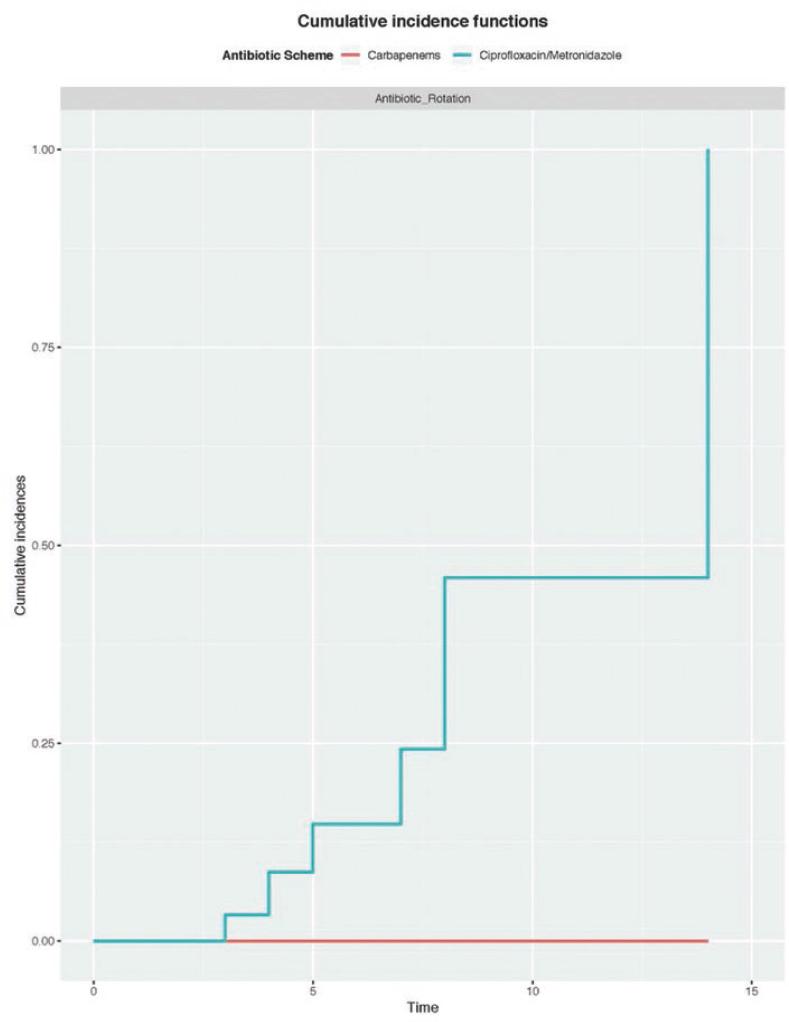
Figure 2 Antibiotic length until rotation (days): ciprofloxacin/metronidazole versus carbapenems (p = 0.012).
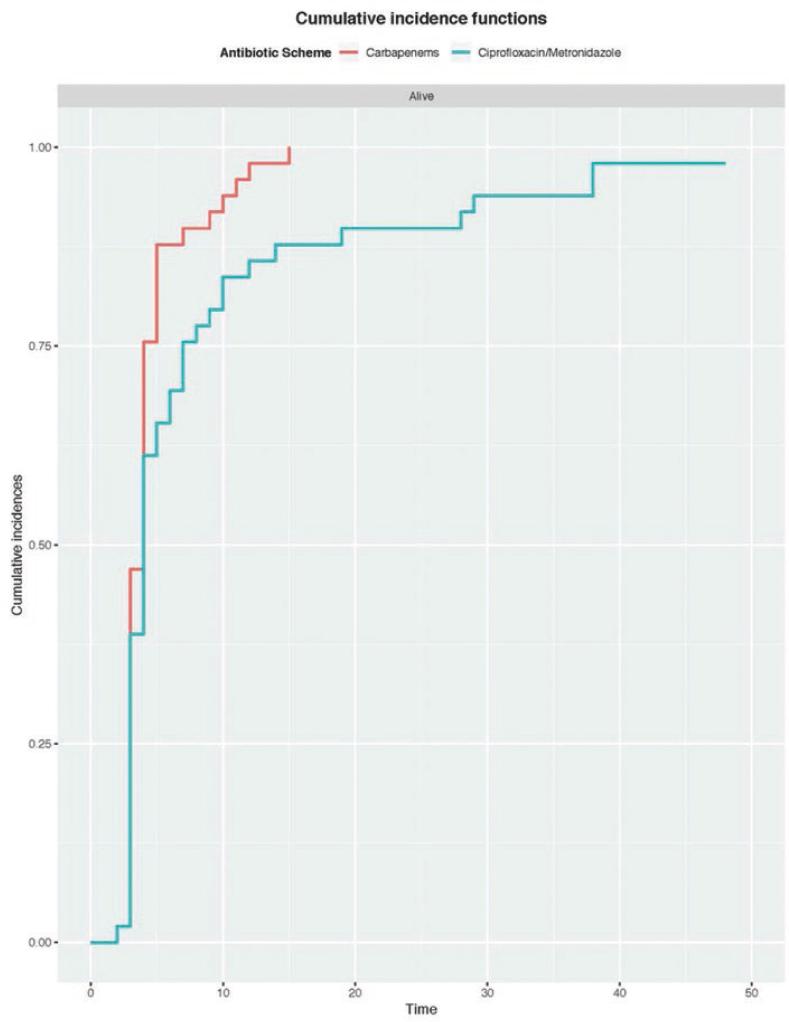
Carbapenems reached a mean hospital stay of 4.45 days (range 2-15) in contrast with ciprofloxacin/metronidazole, 8.29 days (range 2-48) (p = 0.020) (Fig. 3). In carbapenems' subanalysis, the mean antibiotic therapy length and hospital stay in subjects treated with ertapenem were 3.35 days (range 2-11 days) and 4.34 days (range 3-12); in the subjects with meropenem were 3.93 days (range 1-14 days) and 6.99 days (range 2-48). Nevertheless, the use of any of the carbapenems was not associated with a reduced antibiotic therapy length (p = 0.150) or hospital stay (p = 0.109). Table 3 summarizes the characteristics of patients who needed antibiotic rotation or with a hospital stay equal or longer than 14 days.
Table 3 Demographic and clinical characteristics of patients who needed antibiotic rotation or with a hospital stay equal or longer than 14 days
| Patient No. | Age/ Gender | Comorbidities | Type of appendicitis | Type of surgery | Antibiotic | Antibiotic length (days) | Antibiotic rotation | Hospital stay (days) | Hospital discharge condition | Cause of reintervention |
|---|---|---|---|---|---|---|---|---|---|---|
| 18 | 48/M | - | Perforated | Laparotomic | Ciprofloxacin/ metronidazole | 8 | Yes | 28 | Alive | Peritoneal infection |
| 25 | 72/M | - | Perforated | Laparotomic | Ciprofloxacin/ metronidazole | 7 | Yes | 38 | Alive | Loop of bowel |
| 34 | 78/M | Diabetes/ hypertension | Perforated | Laparotomic | Ciprofloxacin/ metronidazole | 14 | Yes | 48 | Alive | Adherolysis |
| 38 | 66/M | Hypertension | Perforated | Laparotomic | Meropenem | 14 | No | 15 | Alive | - |
| 52 | 35/M | - | Perforated | Laparotomic | Ciprofloxacin/ metronidazole | 8 | Yes | 14 | Alive | - |
| 68 | 53/M | Hypertension | Perforated | Laparotomic | Ciprofloxacin/ metronidazole | 12 | No | 19 | Alive | - |
| 84 | 65/M | - | Perforated | Laparotomic | Ciprofloxacin/ metronidazole | 5 | Yes | 29 | Alive | Pyoperitoneum |
| 100 | 36/M | - | Perforated | Laparotomic | Ciprofloxacin/ metronidazole | 4 | Yes | 9 | Alive | - |
| 103 | 19/M | - | Perforated | Laparotomic | Ciprofloxacin/ metronidazole | 3 | Yes | 38 | Alive | Suture dehiscence |
Discussion
The purpose of this study was to compare carbapenems efficacy against to ciprofloxacin/metronidazole in the post-operative management of appendectomy due to CAA (types III-IV). The study shows that carbapenems, compared to ciprofloxacin/metronidazole, achieved a significant reduction in the time of resolution of the infectious disease and in hospitalization stay. It also manages to prevent mediate infections. Antibiotic rotation was necessary only in 7/49 cases from the ciprofloxacin/metronidazole cohort: 5/7 were > 40 years, 7/7 were male, and all of them presented perforated appendicitis resolved through laparotomic surgery (Table 2).
At the time, the use of carbapenems in intra-abdominal infections is cataloged as an alternative empiric treatment. In a recent clinical trial, the efficacy and safety of intravenous (IV) ertapenem, 1 and 1.5 g/day for complicated intra-abdominal infection in adults versus IV ceftriaxone 2 g/day plus IV metronidazole 500 mg/8 h, were compared. After at least 3 days of therapy and satisfactory clinical response, patients could be switched to oral ciprofloxacin/metronidazole. Fifty-nine subjects were randomly assigned to ertapenem 1 g and 51 subjects to ertapenem 1.5 g; 55 subjects were randomly assigned to each compared group. In the healing test, 4-6 weeks after treatment, in the 1 g cohort, 26/31 (84%) of the patients treated with ertapenem and 35/41 (85%) with similar treatment had an evaluation, both clinical and microbiological favorable. Success rates in the 1.5 g cohort were 22/29 (83%) and 24/31 (77%) in the ertapenem and compared groups, respectively.
The adverse events related to the drug were similar in both groups. Ertapenem 1 or 1.5 g once a day followed by optional oral therapy seemed like combined therapy with ceftriaxone plus metronidazole with the same optional oral switch for complicated intra-abdominal infection treatment in adults. Although they were not compared directly at random, ertapenem 1 or 1.5 g efficacy and safety were comparable4. Ertapenem was well-tolerated and had a similar general safety than ceftriaxone plus metronidazole8. Another clinical trial evaluated the state of the art regarding the use of antibiotics in subjects with acute appendicitis undergoing appendectomy in a Chinese tertiary hospital. This study analyzed retrospectively 93 patients that underwent appendectomy and had registered the IV antibiotic used. Subanalysis was done for gangrenous appendicitis, and perforated appendicitis cataloged as advanced appendicitis. The appearance of advanced appendicitis, post-operative complications, and hospital stay were the three main endpoints for the analysis of the results. All subjects received antibiotics before and after the surgical approach. 45/93 (48.1%) subjects received fluoroquinolones, 41/93 (44.1%) received cephalosporins, and 7/93 (7.5%) patients received carbapenems. No statistical significance between antibiotic therapy selection and advanced appendicitis appearance was observed (p = 0.333). Univariate and multivariate analysis did not show a statistical difference between antibiotic therapy selection and post-operative complications (p > 0.05). Median hospital stay in subjects with fluoroquinolone was 2.6 days shorter than those who received cephalosporins (p = 0.0085). A lack of standardization of antibiotic therapy selection criteria in the hospital was concluded. In that study, fluoroquinolones could lead to a shorter hospital stay, but this result could be affected by the number of underlying diseases and the lower severity of the subjects9.
Wilson developed a multicenter, double-blind clinical trial, in which they compared meropenem safety against clindamycin and tobramycin, in 427 subjects form at 13 hospitals in the United States, with some intra-abdominal infection. From this sample, 32% were subjects with complicated appendicitis. The difference in terms of efficacy and hospital stay was statistically significant, but not in the number of complications. It was concluded that meropenem, together with appropriate surgical intervention, constitutes the antibiotic of choice in terms of safety and therapeutic efficacy against secondary intra-abdominal infections6.
The present study has some strengths. It was carried out in a local reference institution; it had a prospective collection of data and design, had a statistically representative sample size, and presented a similar number of cases among those in whom carbapenem versus ciprofloxacin/metronidazole was prescribed. A limitation that can be mentioned was that this work constitutes an observational study. In the absence of randomization, it was not possible to accurately identify many cases, in which the types of carbapenems would be indicated (in the case of imipenem). It did not include other empiric antibiotic therapies, which included aminoglycosides or cephalosporins nor did it have a subanalysis of cost-effectiveness10.
Conclusion
Our observational data let us to conclude that in comparison with ciprofloxacin/metronidazole, carbapenems (ertapenem or meropenem) represent a valid first-line indication for the management of CAA in terms of early infection disease resolution and reduction in hospital stay, especially in patients with determined factors: > 40 years, perforated appendicitis or laparotomic surgery. In this study, no difference between therapeutic outcomes among carbapenems (ertapenem vs. meropenem) was shown. It is recommended to design randomized trials and cost-effectiveness studies which allow establishing the efficacy of carbapenems compared to traditional empiric therapy, mainly in terms of reduction of hospital stay10.











 nueva página del texto (beta)
nueva página del texto (beta)