Introduction
Autologous fat grafting, also known as lipotransference, is a widely-used technique with increased interest over time in both aesthetic and reconstructive surgeries1. However, despite its good results, retaining the volume of the fat grafts is still a challenge. Reported loss of volume ranges from 40 to 90% after lipoinjection2-4, being this the main disadvantage of fat grafting. The loss of volume is evidently due, at least in part, to death of the grafted cells and further resorption. Thus, preservation of the adipocytes during the process of harvesting, processing and injection is of paramount importance.
The processing of the lipoaspirate depends of the preferences of the surgeon. Different processing techniques includes decantation, washing, filtering and centrifugation, among others. Every technique has their own pros and cons, and different level of damage occurs with all the techniques5-8. Despite the chosen method of preparation, with its subsequent quote of damage of the cells, other strategies might improve the rate of viable cells prior grafting.
Refrigeration is a well-known and widely-accepted technique to preserve living tissue, and it has been used to preserve fatty tissue9-11. However, the literature about this subject is sparce and contradictory. Lidagoster and and Cols demonstrated that preserved adipocytes had worse viability compared to non preserved cells11, whereas Bertossi et al reported good results with cryopreserved adipocytes12. Most of the available literature is about cryopreservation of adipocytes for long term and finding the best temperature for that purpose. There are reports of to up to -100 °C13. Other studies reported viability of adipocytes within few hours after cryopreservation, but using liquid nitrogen at -140 °C14
Wang and Cols. studied the viability of adipocytes at 2-8 °C for up to 96 h (24-96 h), reporting similar viability in refrigerated versus non refrigerated samples, although they found decreased metabolic activity overtime15. Matsumoto and Cols preserved lipoaspirates at room temperature for up to 24 h and at 4 °C for 24 to 72 h, to assess morphological changes and metabolic activity13. They found no differences in morphology among the samples, but a decrease in metabolic activity over time.
Since these findings are not applicable to the surgical setting, we designed the present study with the purpose to evaluate the effect of refrigeration (at 4 °C) in the apoptosis and viability of the lipoaspirate in the first 2 h after harvesting.
Methods
20 consecutive patients who underwent liposuction from the abdomen for esthetic reasons were included. Inclusion criteria included adults (= 18 yo), any gender, with a body Mass index (BMI) between 18.5 - 34.9. We excluded patients with history of any chronic disease, with history of any surgery in the abdomen or evidence of infection at the moment of the surgery. Exclusion criteria included samples processed with deviations of the protocol.
The study was approved by the ethics committee of our institution. All patients consented with the processing of their samples.
Lipoaspiration
The lipoaspirate was obtained from the infra-umbilical area using the tumescent technique. Briefly, modified Klein’s solution [1000 cc of Hartmann’s solution (PISA, Guadalajara, Mexico) added to 1 mg of epinephrine (PISA, Guadalajara, Mexico)] was infused through a suprapubic incision, using a 12 G infiltration cannula (Byron, Mentor, Santa Barbara, USA).
Liposuction was performed with a liposuction machine (Sound Surgical Technologies, Louisville, CO) and a 3mm Mercedes cannula (Byron, Mentor, Santa Barbara, USA) at 0.5 bar (-15 mmHg) until 30 ml of the aspirate were obtained.
Five ml of fat were poured in 15 ml Eppendorf tubes (Hauppauge, NY) and decanted for 0, 60, 120 min for further processing. The control group was decanted at room temperature and the refrigerated group was decanted in a refrigerator (Danby Products, Ontario Canada) at 4 °C until processed.
Cellular viability assay
Lipoaspirates were centrifuged at 50 ×g for 5 min immediately after harvesting, at 1 h and 2 h after liposuction. Then, 1 g of the fat fraction was digested with 0.1% type 1 collagenase (US Biological, Salem, MA, U.S.A.) and incubated at 37 °C for 30 min. Digestion was stopped, and the cell suspension was diluted 15:1 with 0.4% trypan blue. Using a Neubauer chamber, adipocytes were counted in 10 random microscopic fields at 40x magnification
Apoptosis assessment
Ten ml of the lipoaspirate was fixed with freshly prepared 4% paraformaldehyde (Sigma-Aldrich, St. Louis, U.S.A.) in phosphate- buffered saline at 0, 60, and 120 min after harvesting for standard histological processing. Adipose tissue apoptosis was determined using TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) assay (TACS TdT in situ fluorescein apoptosis detection Kit; TREVIGEN, Gaithersburg, MD, U.S.A.). The stained nuclei and cells were visualized under a fluorescence microscope. Adipocyte apoptosis in adipose tissue sections was quantified by counting the number of positive nuclei in 10 random microscopic fields at 20x magnification (Fig. 1).

Figure 1 Adipose tissue apoptosis was determined using TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) assay; A: DAPI test. The nuclei are stained in blue; B: Red Light. Used to identify artifacts; C: FITC (fluorescein isothiocyanate). The apoptotic nuclei are stained and visualized under a fluorescence microscope.
Results
20 patients were included in the study. There were no patients excluded or samples eliminated. All patients were female with a median age of 36.5 (21-67) yo. On regard of the viability, at time 0 (immediately after the liposuction) the viability of the samples in the control group (room temperature) was 59.08 ± 24% and 60.96 ± 22% (p = 0-85) in the refrigeration group. At 60 min after harvesting, the viability of the control group was 50.82 ± 21% and 55 ± 32.6% (p = 0.74) in the refrigeration group. At 120 min, the viability of the control group was 42.69 ± 20.85% while in the refrigerated samples was 50.33 ± 21% (p = 0.42). (Fig. 2)

Figure 2 Viability. Refrigerated samples had better viability when compared to samples at room temperature. The difference between the groups increased at each time (p > 0.05) (see text for details).
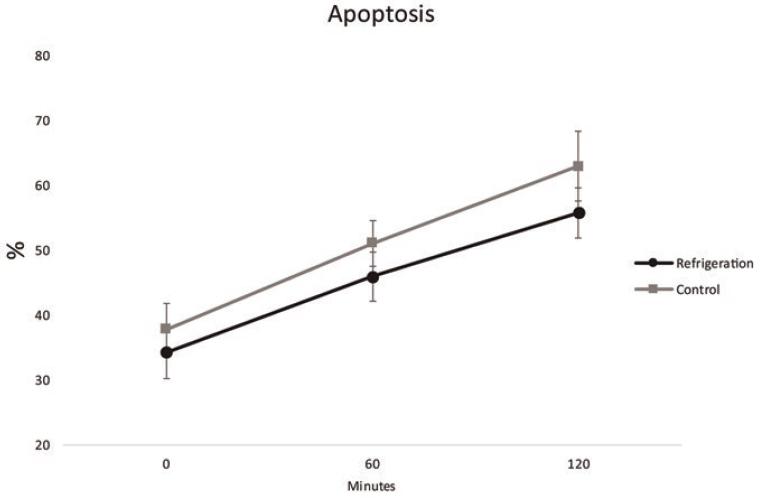
On regard of Apoptosis, the percentage of positive (apoptotic) cells at time 0 was 37.87% ± 9.7% for the control group and 34.28 ± 9.74% (p=0.53). At 60 min the values were 51.11 ± 8.64% for control group and 45.94% ± 9.15% for the refrigeration group (p=0.33). At 120 min, 62.97% ± 13.33% of the cells were apoptotic in the control group versus 55.81 ± 9.45 % in the refrigeration group (p = 0.3) (Fig. 3).
Discussion
Management and preparation of the fat after liposuction are some of the variables included in the fat grafting process, thus, they play an important role in adipocyte survival. Since the viability of the cells decrease overtime once they are deprived from blood supply activity13,16 one of the logical actions to decrease death of the cells would be refrigerate them while they are being collected and prepared for grafting.
Refrigeration, as a method of preservation, is known and used since ancient times17,18 and is one of the basic preservations techniques in laboratories allover the word. Thus, we conducted this study to assess if refrigeration would decrease the death or apoptosis in the adipocytes while they are being prepared for grafting.
As we mentioned previously, there are several studies about cryopreservation of the fat, but most of them were orientated to preserve large amount of fat for a long time, seeking reinjection of the preserved fat in a second surgery. This approach is evidently non-applicable to the common surgical setting where the fat should be grafted within the first couple of hours. Nevertheless, they showed some interesting data that could be pertinent for our study. The studies of Wang and Matsumoto reported short-term viability or morphology of adipocytes, reporting similar results with refrigerated vs non-refrigerated samples13,15.
We decided to study viability and apoptosis of the adipocytes because we believe that this will allow us to see the “full picture” of the damage of the cells. That is, viability assessment might show live cells (metabolically active) but in the process of apoptosis, masking some damage of the cells. Furthermore, the refrigeration, if effective, could decrease mortality or apoptosis of the cells, making relevant to assess both aspects.
The results showed that refrigerated samples had better survival than those left at room temperature, and the difference increased over time. However, the difference was only 5% at 60 min and 8% at 120 min, being not significant (Figure 2). The apoptosis showed similar results, with difference of 6% the 1rst hour and 7% at the 2nd hour. Again, no significance was achieved (p > 0.05) (Fig. 3). With these results, we calculated the sample size required to achieve significance, obtaining a number of 392 patients per group. We believe that the difference between means is so short that there is no point to extend the number of patients in order to achieve significance.
The tendency or our results is that at longer time, more difference between refrigerated vs non-refrigerated samples, according to the existing literature9-13,19. However, we limited the timepoints up to 120 min because that would be a reasonable time for harvesting fat in a common liposuction. Looking for the effect at longer time is beyond the scope of the article.
Based on our results, we believe that refrigeration can improve viability and decrease apoptosis of the adipocytes, but in less than 10% at 2 h. Then, everyone should balance this benefit versus the investment in equipment and implementing of the process for that purpose within the operating room.
It is evident that, in some cases, the liposuction could be extended beyond 2 h; and this protocol did not describe the effect of refrigeration during this time, which would have been interesting. However, we decided to limit our study to 2 h because in a previous study, we demonstrated that viability decreased to 50% at two hours20.
Therefore, we believe that it would not be convenient to decant the aspirate for longer time. Then, we did not consider to extend the study for more than 120 min.











 nova página do texto(beta)
nova página do texto(beta)