Introduction
Pain is an unpleasant sensation associated with sensory and emotional experiences that can cause potential or actual tissue damage.1 Pain after surgery is a maladaptive response that can diminish a patients quality of life.2,3 Acute postoperative pain is an important predictive factor for chronic post-surgical pain, which is observed in 10-65% of patients after surgery. Therefore, controlling pain after surgery is very important.4
The purpose of preemptive analgesia is to reduce postoperative pain, contributing to a more comfortable recovery period and reducing the need for narcotic pain control.1 It refers to the administration of analgesic treatment before surgical incision or tissue injury. It is based on the concept that central and peripheral sensitizations are the major causes of hypersensitivity to pain after injury.1,4,5,6
Agents used in preemptive analgesia include NSAIDs, the neuromodulatory agents gabapentin and pregabalin, acetaminophen, and extended-action local anesthesia. Also, multimodal regimens were developed to reduce opioid consumption and associated adverse effects.7
Preemptive analgesia has been used in orthopedic surgery for total knee and hip arthroplasty, spine instrumentation and fusion, lumbar discectomy, hallux valgus, femoral neck fractures, and knee, shoulder and hip arthroscopy.4
Gabapentinoids drugs (gabapentin and pregabalin) were developed originally as anticonvulsants and subsequently found to be effective in neuropathic and postoperative pain management.8,9,10,11,12 Pregabalin compared to gabapentin has a better pharmacokinetic profile with rapid absorption, higher bioavailability, and less intersubject variability.8,13 Gabapentin has been used in preemptive analgesia for the treatment of thoracotomy, total knee replacement, total hip replacement, mastectomy, lumpectomy, video-assisted thoracoscopic surgery, hand surgery, carpal tunnel surgery, knee arthroscopy, shoulder arthroplasty and shoulder arthroscopy.14 Pregabalin has been used in preemptive analgesia for the treatment of laparoscopic cholecystectomy, laparoscopic hysterectomy, arthroscopic Bankart repair, arthroscopic rotator cuff repair, lumbar fusion surgery, total knee arthroplasty, septoplasty, laparoscopic prostatectomy, radical neck dissection surgery, lower limb orthopedic surgery, molar extraction and endoscopic thyroidectomy.9,15,16,17,18,19,20,21,22,23,24,25,26
Shoulder arthroscopy is a frequent and safe procedure for numerous pathologies and is suited to day-surgery, the difficulty lies in managing pain and the side effects of anesthetic and analgesic drugs.27
The purpose of this review was to assess the efficacy and safety of preemptive analgesia with gabapentinoid drugs for patients undergoing arthroscopic shoulder surgery.
Material and methods
This systematic review was reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. As this is an analysis of previously published articles, participant informed consent and ethical approval are not required.
Search strategy
Two researchers search the relevant studies independently including PubMed (1966-017.12), ScienceDirect (1985-017.12), and the Cochrane Library for potentially relevant studies. Reference lists of all the potential included studies and relevant reviews were hand-searched for any additional trials. Only published articles that had been originally written in English or translated into English were considered. The Mesh terms and appropriate or corresponding terms used in the search and Boolean operators employed were as follows: «gabapentin» or «pregabalin» [Mesh], and «preemptive» and «analgesia» [Mesh] and «arthroscopy» [Mesh] and «shoulder» [Mesh].
Eligibility criteria and study selection
Participants, patients diagnosed with shoulder pathology undergoing arthroscopic surgery, regardless of age and gender.
Intervention, administration of gabapentinoids prior to surgical intervention.
Comparison, placebo administration.
Outcomes, weighted 10-point pain scores after 24 hours after surgery was the primary outcome. Secondary outcomes included opioid consumption 24 hours after surgery, as well as complication rates.
Study design, only RCTs were included.
All searches were limited to human subjects. Eligible studies were assessed independently by 2 authors. A 3rd reviewer acted as a judge if there was any disagreement.
Data extraction
A standard data extraction form was designed using Microsoft Excel, and data were independently extracted from the included studies by 2 reviewers (C-VA and L-VJ). The following data were extracted: author, publication year, sample size, age, gender, drug administered and dosage, and outcomes. The primary endpoints included the following: weighted 10-point pain scores after 24 hours, (0 indicated «no pain» and 10 indicated the «worst imaginable pain»); and incidence of nausea/vomiting, sedation and dizziness. Data in other forms (i.e., median, interquartile range, and mean ± 95% confidence interval (CI)) were converted to the mean ± standard deviation (SD) according to the Cochrane Handbook28 if the data were not reported numerically, we extracted these data using the GetData Graph Digitizer software from the published Figures. All data were extracted by 2 independent reviewers, and disagreements were resolved by discussion.
Quality assessment
The methodological quality of all included trials was independently assessed by 2 reviewers using the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (http://handbook.cochrane.org/). A total of 7 items (random sequence generation, allocation concealment, blinding to the participant and personnel, blinding to the outcome assessment, incomplete outcome, selective reporting, and other bias) were measured. Each of the items was measured as «low risk of bias», «unclear risk of bias», and «high risk of bias». The risk of bias summary and risk of bias graph were obtained using Review Manager (RevMan) [Computer program]. Version 5.3. (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Statistical analyses
Continuous outcomes (weighted 10-point pain scores after 24 hours and opioid consumption at 24 hours) were expressed as the weighted mean differences (WMD) and respective 95% CI. Dichotomous outcomes (occurrence of nausea/vomiting, sedation and dizziness) were expressed as the risk ratio (RR) with 95% CI. Statistical significance was set at p < 0.05 to summarize the findings across the trials. The meta-analysis was calculated by RevMan, version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Statistical heterogeneity was tested using the χ2 test and I2 statistic. When there was no statistical evidence of heterogeneity (I2 < 50%, p > 0.1), a fixed-effects model was adopted; otherwise, a random-effects model was chosen.
Results
Search results
In the initial research, a total of 1695 papers were identified from the electronic databases (PubMed = 2, ScienceDirect = 12, Cochrane Library = 1681); 1 additional record was identified through other sources. Thus, a total of 1696 papers were obtained in the initial search. These bibliographical references were introduced into Mendeley Software (Version 1.19, Elsevier, CA). Duplicates were then removed and 1682 papers were reviewed. After screening the titles and abstracts of these 1682 studies, 1677 papers were excluded because they were irrelevant or did not meet the criteria. Ultimately, 5 clinical studies were included in the meta-analysis. The flow diagram for the included studies is provided in Figure 1.
Study characteristics and bias assessment
The general information of the patients is shown in Table 1. The publication years ranged from 2006 to 2016. The numbers of gabapentinoids and control subjects ranged from 46 to 71. The total gabapentinoids dosages ranged from 150 to 1,200 mg per day. Finally, the follow-up times ranged from 24 to 48 hours. The general characteristics of the included studies were comparable and all studies describe the intent to treat analyses.
Table 1: General characteristics of the included studies.
| Age (years) | Male (%) | Intervention | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Experimental | Control | Experimental | Control | No. Patients | Dose and intervals | Total dose (mg) | Control | Outcomes* | Follow-up (hours) | Study** |
| Adam | 43 | 47 | 66.6 | 69.2 | 53 | Gabapentin 800 mg 2 hours before surgery | 800 | Placebo (n=26) | 1,2,3,4 | 48 | RCT |
| Ahn | 55 | 51 | 43 | 43 | 60 | Pregabalin 150 mg 1 hour before induction of anesthesia | 150 | Placebo (n=30) | 1,2,3,4,5 | 48 | RCT |
| Bang | 56.3 | 59.5 | 34.7 | 39.1 | 46 | Gabapentin 300 mg 2 hour before surgery | 300 | Placebo (n=23) | 1,2,3,4,5 | 24 | RCT |
| Mardani-Kivi | 30.2 | 28.3 | 72.9 | 88.2 | 71 | Gabapentin 600 mg 2 hour before surgery | 600 | Placebo (n=34) | 1,3,4,5 | 24 | RCT |
| Spence | 31.8 | 31.5 | 84.6 | 83.9 | 57 | Gabapentin 300 mg 1 hour before surgery, then 300 mg the first night postoperative and 300 mg BID for 48 hours | 1,800 | Placebo (n=31) | 1,2 | 48 | RCT |
* 1 = VAS at 24 hours, 2 = Total morphine consumption, 3 = Nausea/vomiting, 4 = Sedation, 5 = Dizziness.
** RCT = Randomized controlled trial.
The risk of bias summary and risk of bias graph are provided in Figures 2 and 3, respectively. The risk of bias for selective reporting was unclear in 1 study29 and low in 4 studies.17,30,31,32 The rest all had low risk of bias.17,29,30,31,32
Results of meta-analysis
VAS at 24 hours postoperative
Postoperative VAS scores at 24 hours were reported in 5 studies, and the pooled results indicated that preoperative administration of gabapentinoids was associated with reduced VAS at 24 hours; this corresponded to a reduction of 0.77 points (WMD = -0.77, 95% CI -1.49, -0.05, p = 0.04 (Figure 4)) at 24 hours on a 10-point visual analogue score.
Total morphine-equivalent consumption at 24 hours
Morphine-equivalent consumption at 24 hours was reported in 4 studies, and the pooled results indicated that preoperative administration of gabapentinoids was associated with reduced morphine-equivalent consumption at 24 hours; this corresponded to a reduction of 2.02 mg (WMD = -2.02, 95% CI -3.77, -0.28, p = 0.02 (Figure 5)) at 24 hours of oral morphine equivalent daily dose.
Complications
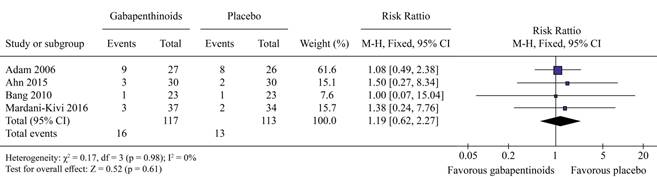
There was no significant difference in the occurrence of dizziness (RR = 1.27, 95% CI 0.75, 2.16, p = 0.37, (Figure 6)) or sedation (RR = 1.19, 95% CI 0.62, 2.27, p = 0.61 (Figure 7). Gabapentinoids can significantly reduce the occurrence of nausea/vomiting (RR = 0.60, 95% CI 0.36, 1.00, p = 0.05 (Figure 8)).
Discussion
The current meta-analysis demonstrated that the use of gabapentinoids is associated with reduced pain scores at 24 hours postoperatively, which is equivalent on a 10-point VAS to 0.77 points, which, even though is statistically significant, may not be clinically relevant. The cumulative morphine-equivalent consumption at 24 hours was reduced in the gabapentinoids group by approximately 2.02 mg. The most important finding of this meta-analysis was that gabapentinoids can reduce the occurrence of nausea/vomiting after arthroscopic shoulder surgery. There was no significant difference in terms of sedation and dizziness.
A major strength of the current meta-analysis was that we comprehensively searched the electronic databases (PubMed, Cochrane Library, and ScienceDirect databases) and calculated the relevant outcomes in a statistically rigorous method. We included RCTs and excluded non-RCTs, and thus the selective risk of bias was largely eliminated. The quality of the included RCTs was high or moderate.
Pooled results indicated that preoperative administration of gabapentinoids was associated with a significant reduction of acute pain at 24 hours following arthroscopic shoulder surgery. These results were in contrast to the Hamilton et al study,33 in which the authors found no evidence to support the routine use of gabapentinoids in the management of acute pain following total knee arthroplasty (TKA) after evaluating 12 RCTs. Mao et al34 performed a meta-analysis of 7 RCTs and found that gabapentinoids were associated with lower pain scores, morphine consumption, and postoperative nausea and vomiting (PONV) following total hip arthroplasty. Liu et al35 performed a meta-analysis of 16 RCTs and found that gabapentinoids were associated with reduced pain scores at 6, 12, 24, and 48 hours. Similarly, gabapentinoids were associated with a reduction in cumulative morphine consumption at 24 and 48 hours. Furthermore, gabapentinoids can significantly reduce the occurrence of nausea, vomiting, and pruritus. There were no significant differences in the occurrence of sedation, dizziness, headache, visual disturbances, somnolence, or urine retention. Jiang et al36 reported that preoperative use of pregabalin was efficacious in the reduction of postoperative pain, total morphine consumption, and the occurrence of nausea following spine surgery. However, the sample size and the number of included studies were limited. Dong et al37 performed a meta-analysis of RCTs on the use of pregabalin for reducing pain after TKA and found that pregabalin was effective in reducing pain intensity, the occurrence of nausea and vomiting but increase the occurrence of dizziness and sedation after TKA. Li et al37 conducted a meta-analysis of 7 RCTs on the use of pregabalin as preemptive analgesia for TKA and THA and found that pregabalin was found to improve pain control at 24 and 48 hours with rest, reduce morphine consumption, improve the knee flexion degree, decrease the incident rate of nausea, vomiting, and pruritus, and increase the incident rate of dizziness after TKA and THA but could not improve the pain control at 72 hours with rest. Yu et al38 conducted a meta-analysis of 7 RCTs on the use of gabapentin and pregabalin as preemptive analgesia for lumbar spine surgery and found that gabapentin and pregabalin can reduce pain intensity at 6, 12 and 24 hours after surgery.
There were several limitations to this meta-analysis: other perioperative pain management protocols were used in all of the studies; shoulder function outcomes were not reported in the included studies and whether better pain control was correlated with preferable shoulder outcomes was unknown; the dosage and interval of gabapentinoid administration differed between the studies, the optimal dose of gabapentinoids required further study; we only identified the published papers about the gabapentinoids versus control groups, so unpublished papers may influence the final results; and none of the studies directly compared gabapentin with pregabalin in arthroscopic shoulder surgery.
Conclusion
This is the first meta-analysis to compare the preoperative use of gabapentinoids versus a placebo for the management of pain after arthroscopic shoulder surgery. Analgesic efficacy and opioid-sparing effects were observed with the administration of gabapentinoids. Additionally, a significant decrease in the risk of nausea and vomiting was associated with the use of gabapentinoids. The optimal dose and dosing intervals of gabapentinoids will require further study.











 nueva página del texto (beta)
nueva página del texto (beta)