Introduction
The normal ligamentum flavum (LF) is a well defined elastic structure containing 80% elastic fibers and 20% collagen fibers.1,2 LF covers the posterior and lateral walls of the spinal canal.3
LF contains the purest form of elastic tissue among ligaments; these elastic fibers decrease with age and are replaced by fibrous tissue.1,3,4
Also decreased in degenerative pathologies in lumbar spine and presented misalignment of the elastic fibers, collagen replacement, alterations in the structure and array of elastic fibers and collagen/elastin ratio and development of calcification over time;1,5 however, none of these studies evaluated all of these parameters in combination.6,7,2
These results were not seen in lumbar disc herniation (LDH) patients. An increase in collagen fibers were observed, but no statistically significant differences were detected between the groups when comparing with lumbar spinal stenosis (LSS); there has been a significant difference in elastic fiber misalignment between the groups.5
All these changes cause reductions in LF elasticity, increased of fibrosis,1,2 and make the LF thickened and to fold into the spinal canal, which may further narrow of the canal,3 and is considered a prodrome of its ossification.8,7
LF thickening is considered a major contributor to the development of LSS and a prodrome of its ossification8,7,9 it compresses the nerve roots of the cauda equina3 and surgical removal of the thickened LF can help treat LSS.3
The purpose of this article is to describe the histopathological changes that occur in some of the degenerative diseases of the lumbar spine, including lumbar degenerative spondylolisthesis (LDS), lumbar spinal stenosis and lumbar disc herniation, and also show the changes presented histopathologically with respect to the age.
Material and methods
All procedures and protocols were conducted in accordance with the principles of Helsinki Declaration, written informed consent was obtained from all participants in accordance with standard operative procedures.
This study included sixty seven patients who were admitted to the Departament of Spine Surgery in our center between 2014 and 2015 and were operated with surgical indications of lumbar spinal stenosis (LSS), lumbar disc herniation (LDH) and lumbar degenerative spondylolisthesis (LDS). In this study all patients fulfilled the following criteria: 1. Age > 18 years, 2. No previous surgery on spine, 3. Diagnosis verified by magnetic resonance scan. Patients with history of osteoporosis, immunosuppression, chronic corticosteroid use, intravenous drug use, fever of unknown origin, history of cancer, unexplained weight loss, or progressive/disabling symptoms were excluded from the study.
LF samples were obtained from the 39 patients who underwent decompressive surgery for LSS, 22 patients who underwent lumbar discectomy for LDH and 6 patients who underwent decompressive surgery and fusion for LDS.
All patients were diagnosed if there were significant MRI findings indicative of these conditions and if clinical manifestations were thought to be compatible with the MRI results. There was no calcification of LF according to preoperative computed tomography scans and X rays.
Outcome parameters
LF materials obtained from patients during surgery were subjected to histopathological analyses. The tissue samples were immediately fixed for 24 hours. Subsequent to preparation with etanol and xylene, tissue was placed in paraffin and sections were cute to 5 μm thickness using a microtome. Tissue preparations were stained with hematoxylin and eosin, and were evaluated by two pathologists who were blinded to the nature of the groups. Specimens were examined with regard to chondroid metaplasia, calcification, fragmentation of collagen fibers, cystic degeneration, fibrillar appearance, hypercellularity, and the presence of others degenerative changes.
Statistical analysis
Data were analyzed using the IBM Statistical Package for Social Sciences ver. 20 (SPSS Inc., Chicago, IL, USA). Parametric tests were applied to data of normal distribution and nonparametric tests were applied to data of questionably normal distribution. Continuous data were presented as mean ± standard deviation or median (minimum-maximum), as appropriate. We used ANOVA to analyze variances between groups. All differences associated with a chance probability of ≤ 0.05 were considered statistically significant.
Results
Sixty-seven patients (33 males, 34 females) met the eligibility criteria for the study. Of these patients whose charts were reviewed, the mean age was 54.37 years (range, 19-87 years).
In the analysis by age group, we found that the highest percentage of sample is within the group between 44 and 65 years; in the group of LDH are the youngest patients with a p = 0.0001 (Table 1).
Table 1: Description of the sample.
| Diagnosis | |||||
|---|---|---|---|---|---|
| Parameter | Lumbar spinal stenosis | Lumbar disc herniation | Lumbar degenerative spondylolisthesis | Total | p |
| n (%) | 39 (58.2) | 22 (32.8) | 6 (9) | 67 (100) | |
| Age in years M (SD) | 61.49 (12.60) | 39.91 (11.15) | 61.17 (9.45) | 41.61 (8.95) | 0.980 |
| % | (%) | (%) | (%) | ||
| Group 1 (19-43 years) | 0.0 | 4.5 | 0 | 4.5 | |
| Group 2 (44-65 years) | 47.8 | 28.4 | 7.5 | 83.6 | |
| Group 3 (66-87 years) | 10.4 | 0.0 | 1.5 | 11.9 | |
| Gander | |||||
| Men | 43.6 | 63.6 | 33.3 | 49.3 | 0.0001 |
| Women | 56.4 | 36.4 | 66.7 | 50.7 | |
| Histopathological changes | |||||
| Chondroid metaplasia | 25.6 | 22.7 | 16.7 | 65.0 | 0.881 |
| Calcification | 23.1 | 36.4 | 0.0 | 59.5 | 0.169 |
| Fragmentation of collagen fibers | 23.1 | 45.5 | 66.7 | 89.8 | 0.045 |
| Cystic degeneration | 2.6 | 0.0 | 33.3 | 35.9 | 0.001 |
| Fibrillar appearance | 10.3 | 4.5 | 50.0 | 64.8 | 0.009 |
| Hypercellularity | 0.0 | 0.0 | 16.7 | 16.7 | 0.006 |
On the top we observed distribution by group according to the age, we observed that 83.6% of the sample corresponds to the group between 44 and 65 years of age, the highest percentage in LDH group is in the youngest group (p = 0.0001) and analysis by gender. In the botton we described histopathological changes presented in the different groups; the LSS group has more prevalence of chondroid metaplasia; the LDH group has more prevalence of calcification; and the LDS group has more fragmentation of collagen fibers, cystic degeneration, fibrillar appearance and hypercellularity.
Groups did not differ from each other regarding age (p = 0.980), but differ in gender (p = 0.0001).
The most frequent histopathological changes were hyalinization and fragmentation of collagen fibers occur in 34% of the total samples, neovascularization in 40.3% and irregular arrangement of elastic fibers is the most prevalent change with 56.7%.
A multiple linear regression was performed to evaluate the effect of age and gender with the presence of the most important changes in the analysis (hyalinization, fragmentation, irregular fiber arrangement and neovascularization) and those were not predictors in the mentioned changes (Figures 1 to 7).
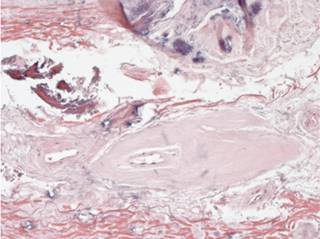
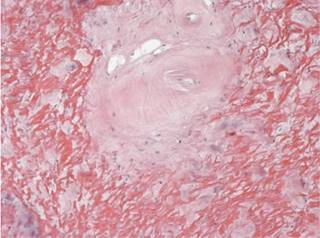
Figure 1: Immersed in the yellow ligament there is accentuation of the vascular pattern with. In this case with hyalinization of the vascular walls.

Figure 3: Areas of mononuclear inflammatory infiltrate associated with fragmentation of elastic fibers are observed.
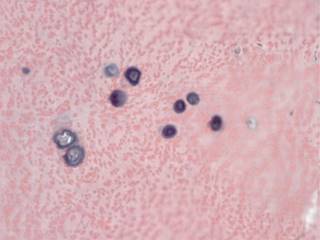
Figure 4: There are areas of calcification immersed in the elastic fibers, which are seen round, basophilic and concentric, which resemble bodies of psammoma.

Figure 5: In the ligament flavum there are acellular basophilic amorphous nodular zones, which give them a hyalinized appearance.
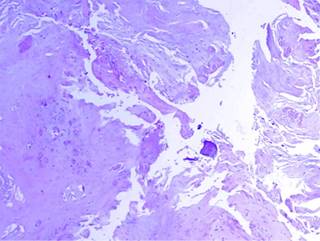
Figure 7: On the surface there are areas of frayed appearance, which correspond to the areas of fibrillar degeneration.
There is a difference in the presence of certain changes in the flavum ligament according to the diagnosis, being statistically significant for fragmentation of collagen fibers (p = 0.045), cystic degeneration (p = 0.001), fibrillar appearance (p = 0.007) and hypercellularity (p = 0.005).
The group of LDS presented separation of collagen fibers, cystic degeneration, fibrillar appearance and hypercellularity with p value < 0.05 (Table 1). There is an association between degenerative spondylolisthesis and the separation of collagen fibers. We found the LDH group had fragmentation of collagen fibers, fibrillar appearance, despite belonging to a younger age. The LSS group showed fragmentation of collagen fibers, cystic degeneration, fibrillar appearance and hypercellularity (Table 1).
Discussion
We sought to compare the histopathological changes found in patients with LSS, LDH, LDS. Altun Idiris found that calcification was not detected (0.00 ± 0.00) in the discectomy group,5 in our analysis calcification was detected in 36.4% of the samples of LDH group. In our study the groups did not differ with regard to mean calcification, chondroid metaplasia, (p=0.881 and p=0.169, respectively). But differ in fragmentation of collagen fibers, cystic degeneration, fibrillar appearence and hypercellularity, most of this changes were seen in LDS, these results could be cause by instability rather than age o degenerative disease in the spine.
The mean age of patients in the stenotic group did not differ from that of the discectomy group.5 However we found the highest percentage in LDH (72.7%) group is in the youngest group (19-43 years old). And also we observed some degenerative changes in the LDH group like fragmentation of collagen fibers in 45.5% and fibrilar appearence in 4.5%. we concluded the same that Postacchini no peculiar changes occur in patients with disc herniation, and showed similar features to those of ligaments from control subjects of similar ages.10
In spinal stenosis, fibrotic changes, chondroid metaplasia, and calcification reduce the elasticity of the ligaments, wich may thus bulge into the spinal canal in the standing position even if they are normal in thickness, which could explain why we found statistically significant differences between the groups in age and histopathological changes.10,11
Debility of the study is the sample, is small and we don´t have the same number of patients in each group and we don’t have a control group. Because of these limitations, our results should be interpreted with caution. In addition, it is important to note our analysis did not focus on the mechanism of the development of spinal stenosis.
Degeneratives changes in LF may occur as a result of elastic fiber misalignment, the instability in DS cause the degenerative changes observed in LF. Further studies determining the pathogenesis of LSS are needed.
Conclusions
LDS present more degeneratives changes like fragmentation of collagen fibers, cyst degeneration and fibrillar appearance, than the other groups and these is caused by instability.
The group of LDH present degenerative changes despite belonging to a younger age.
There is not evidence of cellular hyperthophy in the histhopatological analyses, thickening of the LF can be seen by bulking of LF followed by collapse of motion segment.











 nueva página del texto (beta)
nueva página del texto (beta)