Background
Giant cell tumor is a bone tumor that has been known since the 19th century when Sir Astley Cooper in 1818 called this condition fungus medullary exostoses,1 and later in 1940, Jaffe, Lichtennstein and Portis redefine it to as it is now known,2 which according to the classical definition of Mario Campanacci, is an intramedullary bone tumor with specific preference by age and location, composed of mononuclear cells and giant cells that resemble osteoclasts and presenting a growth potential variable and unpredictable.3 This lesion represents approximately 5% of all primary bone neoplasms,4,5,6,7,8,9 and 20% of benign bone tumors.5,6 This tumors occur most frequently between the second and fourth decades of life.4,6,7 The most common locations are in descending order: distal femur, proximal tibia and distal radius,3,5 all of them in metaphyseals zones and in intimate contact with the subchondral bone.
The clinical manifestations of the giant cell tumor of bone are mainly local pain with increased volume and hypersensitivity, and may also exist pathological fracture secondary to the cortical thinning caused by the lesion.10
From the radiological point of view it is an osteolytic lesion with a pattern of destruction usually geographical, expansive and eccentric, which may present septa in the interior. Due to rupture of the cortical, a soft tissue shadow can be observed secondary to tumor invasion into the surrounding tissues. Campanacci et al described a system of radiological staging11,12 in which a clinical-radiological correlation is noted:
Stage I: they are lesions that do not distort or perforate the cortical,11 which is indeed thinned. The tumor is usually well demarcated by a fine more or less well-defined halo. Clinically they’re lesions with minimal symptomatology, or with an indolent course. It is estimated that only 10% of giant cell tumor of bone are diagnosed at this stage.3 They are usually latent lesions, i.e. they correspond to grade 1 Enneking classification.13
Stage II: are those in which an image clearly osteolytic is seen which also distorts or expands the cortex, but even without spread to peripheral soft tissue. There´s no any sclerosus halo settling to the lesion, however it is possible to specify the transition between the sick and the healthy bone. There is established symptomatology and its growth is stable. Approximately 70% of cases are diagnosed at this stage.3 Usually are active lesions that clinically correspond to grade 2 of Enneking.13
Stage III: they are osteolytic lesions that penetrate the cortex and extends to the surrounding soft tissues; sometimes a phantom bone imaging its observed. There is not an accurately lesion limits, being in this type of injury where the highest rate of local recurrence occurred. These lesions are often associated with pathologic fractures.3 Are aggressive tumors with a fast growth that clinically correspond to a grade 3 of Enneking.13
There are several treatment options for giant cell tumors, we can however summarize them in intralesional resections (radiological stages I and II of Campanacci lesions), wide resections (radiological stage III of Campanacci) and radical procedures. This last alternative treatment is reserved for aggressive lesions that involve a great amount of soft tissue, important cutaneous commitment, and/or major neurovascular invasion. Giant cells tumors of the distal end of the radius has a particular and well known tendency of local recurrence, in addition to being the one that more easily develop metastasis or lung histologically benign implants (in relation to the rest of the locations).
Known as anterolateral thigh flap to the septocutaneous or musclecutaneous flap, based on the perforator artery of the descending portion of the lateral femoral circumflex artery.14,15,16 It was first described in 1984.
Since its initial description, the anterolateral thigh flap has gained popularity in its use as a flap of soft tissue for reconstruction of regional and distance defects.15
The marking of the flap is based on the location of the skin vessels supplying the territory of the flap. The anatomical references include the anterior superior spine iliac and the side edge of the upper pole of the patella; the flap is focused at the midpoint of a longitudinal line which unites them. A circle of 3 cm radium defines the area in which skin vessels, septocutaneous vessels or the perforator musculocutaneous come out; subsequently It is designed the cutaneous shovel around the defined dermal vessels. The dimensions of the flap can be as large as 35 cm long and 25 cm wide.14,15 The versatility of this flap is such that can be designed with dermal portion, fascia and even muscle. The fascia lata possessing vascular pedicle that can be used in tendon reconstruction.16 Also used in the treatment of shrink scars and contractures in the hand, as well as to treat large defects of soft tissue with loss of blood vessels, in which case the pedicle is lodged in the vascular defect with satisfactory results.17,18 In what refers to oncological origin reconstructions, the anterolateral thigh flap provides a proper tissue coverage with special characteristics, as it might be used in composite form, including the reconstruction of tendons, support structures, and through the femoral cutaneous nerve, nerve structures that will allow us to preserve the sensitivity.19,20
Case presentation
Male patient 23-year-old without any genetic and personal background of importance. He began his condition in August 2015 with volume increase at the level of the left wrist, as well as light local hyperthermia and progressive limitation to the normal ranges of joint mobility. Through imaging studies it is detected a lytic, expansive, extracompartimental and heterogeneous component bone tumor at expense of the distal radius (Figure 1). Thick needle biopsy is performed, and already with histopathological report of giant cell tumor of bone, is referred to our medical unit.
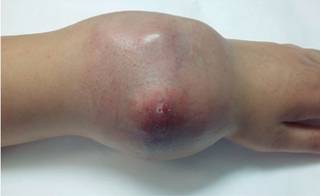
The patient was received by the plastic surgery department. Physical examination at that time suggest a great mass of neoplastic tumor lobulated that severely compromise the skin cover of the region without exposure of the mass (Figure 2). The movements of the wrist are abolished by trigger pain and disability secondary to the extent growth of the neoplasm. Radiologically is already with a phantom bone sign.21

Figure 1: X ray showing a lytic and destructive lesion (phantom bone sign) in the distal radius consistent with a giant cell tumor.
The patient was surgically scheduled for extend resection including cutaneous coverage for the poor condition of this and the scar from previous biopsy, as well as reconstruction through ulnar centralization and ulnar-carpal arthrodesis directed the third ray, plus anterolateral contralateral thigh flap.
Surgical technique
Prior identification trough vascular Doppler of the radial neurovascular package and measurement of dimensions of the skin to be included in the surgical specimen, tissue is then drawn and then approached including a spindle of damaged skin (Figure 3) (en bloc resection using the criteria of broad Enneking margins) continuing with the completion of fasciocutaneous flaps from the remnants of the cutaneous cover in the region of the forearm and wrist of the patient.
Once resected most of the tumor (Figure 4), and preserving a layer of healthy muscle tissue, proceeds to make proximal radial resection of approximately half of the length being gradually distally released the surgical specimen. Once identified the tendons trapped at the interior of the tumor mass were identified, and resected at the wrist, on the tumor edge, being released from it, and then retracted and preserved following the path of their respective pulley, then the complete resection of the distal radius is done, including the first carpal line.
For 15 minutes a surgical lavage is performed using hydrogen peroxide within the surgical bed, the ulcer is then centralized and an ulnar-carpus arthrodesis is completed. For this purpose A 9 holes 3.5 DCP plate was used (Figure 5). Extensors tendon repaired were carried out. Simultaneously to this late stage of the procedure the anterolateral thigh flap was performed; a 16 x 10 cm skin flap was harvested, including a fascia lata compound for extensor retinaculum reconstruction using microsurgical approach (Figure 6).
Once the procedure has been complete, a cast anteriorly placed was used, and an intensive program of physical therapy and rehabilitation was established.
Two months after procedure the patient is functionally with flexion and extension full of fingers 2 to 5, with limitation to the full flexion of the left thumb with oral intake of risedronate. Currently no evidence of tumoral activity and with radiological arthrodesis achieved.
Discussion
Giant cell tumor of bone is a benign neoplasm of locally aggressive behavior that can even require radical procedures in extreme cases. Early diagnosis is the key to less aggressive treatments and with a lower functional deficit. Control of the disease is the priority. The distal radius is one of the classic locations for giant cell tumors, followed by the knee. The great mobility of the wrist and hand, is the importance of this anatomical region. The circumstances which may lead to the development of large tumors vary from educational causes and health systems, to those inherent to the histological nature of the neoplasm. Current medicine requires multidisciplinary approaches which provide a combination of skills for the patient outcome and the innovation in terms of new therapeutic modalities. The case presented here is exposed to a neoplasm of fast growth in a critical area for upper extremity function. An alternative treatment, nothing reprehensible might have been the amputation of the affected segment, the great bone damage and the condition of soft tissue could well justify it. In this patient, it was possible to focus on a multidisciplinary strategy and fortunately the outcome has been satisfactory. Ulnar-carpus arthrodesis result in the loss of mobility of the wrist and the forearm pronosupination. If this fusion is in the right position, it is possible to preserve a greater degree of function far superior to an amputation and its external prostheses. The function of the hand as such is practically preserved. The skin coverage obtained through the anterolateral thigh flap, has been excellent.











 nueva página del texto (beta)
nueva página del texto (beta)