Introduction
Currently, the most important transgenic crops worldwide are: soybeans, maize, cotton and canola. Soybean ranks first, followed by maize, cotton and canola with 105, 60.9, 25.7 and 10 million ha, respectively (ISAAA 2019, Voora et al. 2020). Because of the agronomic benefits that the use of genetically modified seeds represents, GM crops have gained acceptance in some countries in such a way that the estimated global area of transgenic crops has increased from 1.7 million ha in 1996 to 273.4 million at the present time (Brookes 2022). However, use of these transgenic organisms has generated an intense debate that goes from the scientific field to economic, social, political, ethical and moral levels (Sharma et al. 2022). Maize is the most important agricultural crop in Mexico, from the point of view of food, as well as industrial, political and social sector (Ibarrola-Rivas et al. 2020), as referred to the different archaeological, genetic, biochemical and historical studies that allow understanding the use of corn in the Mexican history, and evolutionary history of this cereal (Erenstein et al. 2022), that helps to understand the diversity of endemic maize varieties in this country (Orozco-Ramírez et al. 2016, Fonseca et al. 2023).
Worldwide, only 20% of maize production goes to human consumption, and 80% is used as animal feed or industrial raw material (Grote et al. 2021, Erenstein et al. 2022), however, in Mexico, 68% of its maize production is used for human consumption (Ibarrola-Rivas et al. 2020, Arce et al. 2020). Based on these antecedents and adding that Mexico has a wide diversity of natural conditions that provides numerous environments for maize growth (Vidal and Brusca 2020, Grote et al. 2021). In addition, these particular diverse environmental conditions have allowed to developing by selection and interaction with environment, very specific populations, which are commonly referred as maize varieties, a term used to refer to a population native of the community, region, state or country, differing from a foreign material as a hybrid or an improved variety. Local varieties are heterogeneous populations of plants that are differentiated by farmers based on their color, texture, and shape of the grain, shape of the ear, crop cycle and use. They are maize populations developed by farmers for many years, through the management and conservation of seeds and genes through a system of exchange year after year. Maize varieties (hybrid or improved varieties) is also considered to be the set of plants that are the product of a natural or artificial crossing (crossed by farmers, by breeders or both) with an improved material, respecting that this set of plants. It is constituted with 75% of the original variety’s genetic material and 25% of the improved material (Salgotra and Chauhan 2023).
Maize in a large percentage is an open pollinated crop, which facilitates its crossing between varieties (Guzzon et al. 2021, Li et al. 2023) and with some of the species considered as ancestors as Zea perennis (Lohn et al. 2021), but also with commercial hybrids (Eze et al. 2020). This situation has led to a genetic contamination by transgenes in the natural ecosystems of Mexico, as it has been documented. Since 2001, there are isolated reports that indicate the presence of GM sequences in maize varieties in Oaxaca and some others Mexican states (Quist and Chapela 2001, Cleveland et al. 2005, Serratos-Hernández et al. 2007, Mercer and Wainwright 2008, Piñeyroet al. 2009, Orozco-Ramírez et al. 2016, Rendón-Aguilar et al. 2019). Based on these antecedents, and in view of the degree of contamination of these native maize with transgenic genes is unknown in other Mexican states, as well as the transgenic sequence (s) that have been introduced. The objectives of this study were to determine the level of introgression of transgenes into maize varieties of Puebla, Oaxaca, Jalisco and Michoacán States, to estimate of frequencies of maize contamination and to identify the main transgenic events transferred to the maize varieties.
Materials and methods
Collection of maize varieties
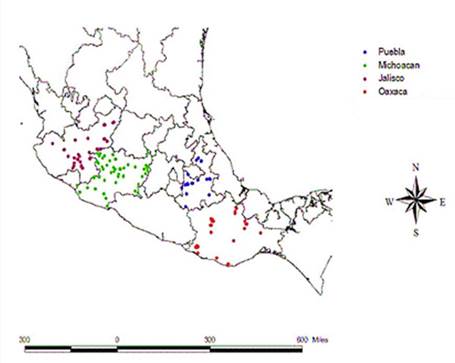
Samples were collected by field trips to rural community from the states of Puebla, Jalisco, Michoacán and Oaxaca. Each sample weighted 1000 g of composite seed and was obtained from participate producer which were randomly chosen. In this study, a total of 215 maize local varieties were obtained, 53 out of 215 seed samples were collected from Jalisco, 96 samples from Michoacán, 46 samples from Oaxaca and 20 samples from Puebla State (Table 1). Each sampling site was geo-referenced in terms of altitude, latitude and longitude. Seed from each sample was placed for conservation in the maize germplasm bank of the Universidad Autonoma Agraria Antonio Narro.
Table 1 Localities and maize genotypes collected from Jalisco (J), Michoacán (M), Oaxaca (O) and Puebla (P) states.
| No | Key | Genotype | Locality | Latitude (N) | Longitude (W) | Altitude (masl) |
|---|---|---|---|---|---|---|
| 1 | J1 | Celaya 2010 (C-2) | Jamay | 20°17’ 17.60" | 102°41’ 39.46" | 1530 |
| 2 | J2 | Celaya 2010 (3) | Jamay | 20°17’ 18.44" | 102°40’ 35.91" | 1528 |
| 3 | J3 | Celaya 2010 (4) | La Barca | 20°18’ 14.07" | 102°31’ 57.01" | 1535 |
| 4 | J4 | Celaya 2010 (5) | La Barca | 20°17’ 17.89" | 102°31’ 45.67" | 1538 |
| 5 | J5 | Celaya 2010 (6) | La Barca | 20°17’ 20.72" | 102°33’ 14.11" | 1533 |
| 6 | J6 | Celaya 2010 (7) | Jesus Maria | 20°36’ 33.29" | 102°12’ 57.00" | 2121 |
| 7 | J7 | Celaya 2010 (8) | Arandas | 20°41’ 54.89" | 102°20’ 0.67" | 2045 |
| 8 | J8 | Celaya 2010 (10) | Arandas | 20°41’ 47.11" | 102°21’ 39.20" | 2054 |
| 9 | J9 | Celaya 2010 (11) | Arandas | 20°41’ 10.49" | 102°20’ 23.92" | 2063 |
| 10 | J10 | Celaya 2010 (12) | San Juan de los Lagos | 21°14’ 34.26" | 102°21’ 40.89" | 1870 |
| 11 | J11 | Celaya 2010 (15) | San Juan de los Lagos | 21°16’ 1.50" | 102°21’ 2.47" | 1750 |
| 12 | J12 | Celaya 2010 (16) | San Juan de los Lagos | 21°15’ 22.04" | 102°18’ 52.99" | 1784 |
| 13 | J13 | Celaya 2010 (17) | Lagos de Moreno | 21°21’ 4.92" | 101°54’ 54.31" | 1875 |
| 14 | J14 | Celaya 2010 (18) | Lagos de Moreno | 21°20’ 25.71" | 101°56’ 23.89" | 1868 |
| 15 | J15 | Celaya 2010 (19) | Jesus Maria | 20°36’ 44.99" | 102°13’ 35.45" | 2047 |
| 16 | J16 | Celaya 2010 (20) | Lagos de Moreno | 21°21’ 3.65" | 101°54’ 19.99" | 1883 |
| 17 | J17 | Celaya 2010 (22) | San Diego | 20°9’ 38.30" | 103°3’ 25.44" | 1541 |
| 18 | J18 | Celaya 2010 (23) | Mazamitla | 19°54’ 55.78" | 103°0’ 44.19" | 2237 |
| 19 | J19 | Celaya 2010 (24) | San Diego | 20°10’ 20.59" | 103°3’ 43.75" | 1528 |
| 20 | J20 | Celaya 2011 (25) | Atoyac | 20°0’ 45.84" | 103°31’ 15.87" | 1356 |
| 21 | J21 | Celaya 2010 (26) | Mazamitla | 19°54’ 53.76" | 103°1’ 41.44" | 2197 |
| 22 | J22 | Celaya 2010 (27) | Tala | 20°38’ 54.70" | 103°42’ 56.39" | 1317 |
| 23 | J23 | Celaya 2010 (28) | Tala | 20°39’ 0.69" | 103°43’ 24.04" | 1310 |
| 24 | J24 | Celaya 2010 (29) | Tala | 20°39’ 39.62" | 103°43’ 16.07" | 1312 |
| 25 | J25 | Celaya 2010 (30) | Tapalpa | 19°56’ 25.18" | 103°45’ 33.94" | 2039 |
| 26 | J26 | Celaya 2010 (32) | Tapalapa | 19°56’ 37.41" | 103°46’ 7.16" | 2038 |
| 27 | J27 | Celaya 2010 (34) | Atoyac | 20°0’ 35.07" | 103°31’ 25.77" | 1353 |
| 28 | J28 | Celaya 2010 (35) | Sayula | 19°52’ 22.70" | 103°34’ 57.37" | 1365 |
| 29 | J29 | Celaya 2010 (37) | Sayula | 19°53’ 45.17" | 103°35’ 49.82" | 1359 |
| 30 | J30 | Celaya 2010 (38) | Gomez Farias | 19°47’ 56.00" | 103°28’ 55.90" | 1516 |
| 31 | J31 | Celaya 2010 (39) | Zapotiltic | 19°37’ 32.73" | 103°26’ 9.10" | 1370 |
| 32 | J32 | Celaya 2010 (40) | Zapotiltic | 19°37’ 10.96" | 103°25’ 34.27" | 1335 |
| 33 | J33 | Celaya 2010 (42) | Tuxpan | 19°32’ 48.94" | 103°22’ 8.36" | 1130 |
| 34 | J34 | Celaya 2010 (43) | Tuxpan | 19°32’ 48.28" | 103°23’ 0.04" | 1124 |
| 35 | J35 | Celaya 2010 (45) | Techaluta | 20°4’ 26.72" | 103°33’ 25.90" | 1470 |
| 36 | J36 | Celaya 2010 (46) | Tamazula | 20°0’ 58.77" | 104°3’ 3.71" | 929 |
| 37 | J37 | Celaya 2010 (47) | Techaluta | 20°4’ 40.19" | 103°33’ 28.47" | 1486 |
| 38 | J38 | Celaya 2010 (48) | Tamazula | 20°1’ 13.20" | 104°3’ 8.47" | 938 |
| 39 | J39 | Celaya 2010 (49) | Zapotitlan | 19°32’ 58.41" | 103°49’ 13.04" | 1098 |
| 40 | J40 | Ancho de color | Atengo | 20°16’ 16.23" | 104°14’ 40.43" | 1425 |
| 41 | J41 | Ancho blanco | Atengo | 20°15’ 58.93" | 104°14’ 25.38" | 1406 |
| 42 | J42 | Elotes occidentales | Atengo | 20°16’ 13.88" | 104°14’ 29.07" | 1432 |
| 43 | J43 | Amarillo Varieties | Tapalpa | 19°57’ 11.55" | 103°45’ 50.05" | 2114 |
| 44 | J44 | Dulce de jalisco | Navidad, Mascota | 20°31’ 36.12" | 104°46’ 29.61" | 1236 |
| 45 | J45 | Amarillo | Atengo | 20°43’ 3.65" | 103°7’ 12.37" | 1700 |
| 46 | J46 | Coamilero | Atengo | 20°16’ 18.50" | 104°14’ 39.88" | 1426 |
| 47 | J47 | Tabloncillo perla | Luis A. Arciga, Atengo | 20°16’ 36.77" | 104°13’ 53.33" | 1449 |
| 48 | J48 | Bofo | Atengo | 20°16’ 30.78" | 104°13’ 55.47" | 1440 |
| 49 | J49 | Tabloncillo pequeño | Alista, San Gabriel | 19°37’ 53.66" | 103°47’ 12.89" | 1344 |
| 50 | J50 | Maíz dulce de Jalisco | Alista, San Gabriel | 19°37’ 55.96" | 103°47’ 34.75" | 1338 |
| 51 | J51 | Maíz dulce de Jalisco | Alista, San Gabriel | 19°38’ 7.92" | 103°46’ 55.58" | 1369 |
| 52 | J52 | Maíz dulce de Jalisco | Alista, San Gabriel, | 19°37’ 46.56" | 103°47’ 35.42" | 1317 |
| 53 | J53 | Amarillo de ocho | Plan de las Flores | 19°44’ 58.48" | 103°45’ 35.22" | 1293 |
| 54 | M1 | Argentino | Buenavista | 19°11’ 55.24" | 102°35’ 16.77" | 432 |
| 55 | M2 | Argentino | Periban | 19°30’ 45.19" | 102°25’ 22.86" | 1686 |
| 56 | M3 | Argentino | Cotija | 19°47’ 50.68" | 102°41’ 27.85" | 1649 |
| 57 | M4 | Argentino | Villamar | 20°1’ 17.44" | 102°35’ 24.18" | 1571 |
| 58 | M5 | Argentino | Jocotepec | 20°16’ 42.93" | 103°25’ 37.81" | 1537 |
| 59 | M6 | Argentino | Vista Hermosa | 19°45’ 20.62" | 102°37’ 35.35" | 1625 |
| 60 | M7 | Argentino | Pajacuaran | 20°7’ 5.54" | 102°33’ 2.75" | 1527 |
| 61 | M8 | Argentino | Venustiano Carranza | 20°7’ 6.49" | 102°39’ 36.33" | 1530 |
| 62 | M9 | Argentino | Tanganmandapio | 19°57’ 8.47" | 102°26’ 38.89" | 1713 |
| 63 | M10 | Argentino | Cotija | 19°47’ 57.64" | 102°42’ 3.65" | 1717 |
| 64 | M11 | Celaya | Cotija | 19°48’ 0.99" | 102°42’ 11.61" | 1717 |
| 65 | M12 | Celaya | Huaniqueo | 19°17’ 59.68" | 101°40’ 42.18" | 2273 |
| 66 | M13 | Celaya | Huaniqueo | 19°18’ 2.46" | 101°41’ 12.31" | 2276 |
| 67 | M14 | Chalqueño | Epitacio Huerta | 20°8’ 10.63" | 100°17’ 4.45" | 2473 |
| 68 | M15 | Chalqueño | Angangueo | 19°36’ 33.89" | 100°17’ 33.98" | 2604 |
| 69 | M16 | Chalqueño | Ocampo | 19°35’ 3.43" | 100°20’ 39.46" | 2296 |
| 70 | M17 | Conico | Morelos | 18°40’ 46.67" | 99°6’ 4.78" | 951 |
| 71 | M18 | Conico | Morelos | 19°13’ 10.75" | 101°42’ 24.42" | 1960 |
| 72 | M19 | Conico | Morelos | 19°13’ 11.96" | 101°42’ 9.11" | 1959 |
| 73 | M20 | Conico | Huiramba | 19°32’ 30.98" | 101°26’ 7.10" | 2173 |
| 74 | M21 | Conico | Patzcuaro | 19°30’ 19.67" | 101°37’ 47.10" | 2194 |
| 75 | M22 | Conico | Patzcuaro | 19°31’ 15.74" | 101°37’ 21.50" | 2097 |
| 76 | M23 | Elotes occidentales | Caurio | 19°53’ 33.42" | 101°49’ 28.86" | 2164 |
| 77 | M24 | Elotes occidentales | Caurio | 19°53’ 49.57" | 101°49’ 59.41" | 2126 |
| 78 | M25 | Elotes occidentales | Caurio | 19°53’ 36.49" | 101°49’ 47.83" | 2142 |
| 79 | M26 | Elotes occidentales | Caurio | 19°53’ 27.89" | 101°49’ 28.56" | 2161 |
| 80 | M27 | Elotes occidentales | Caurio | 19°54’ 16.98" | 101°48’ 48.50" | 2250 |
| 81 | M28 | Ancho | Charapan | 19°38’ 43.64" | 102°14’ 37.76" | 2273 |
| 82 | M29 | Ancho | Tlajomulco | 20°28’ 28.02" | 103°26’ 18.97" | 1590 |
| 83 | M30 | Ancho | Jungampeo | 19°29’ 33.93" | 100°29’ 24.99" | 1581 |
| 84 | M31 | Ancho | Jungampeo | 19°29’ 37.21" | 100°29’ 17.80" | 1603 |
| 85 | M32 | Mushito | Madero | 19°26’ 44.58" | 100°19’ 4.16" | 2163 |
| 86 | M33 | Mushito | Ario | 20°1’ 40.22" | 102°20’ 19.06" | 1564 |
| 87 | M34 | Mushito | Tacambaro | 19°13’ 32.38" | 101°27’ 19.27" | 1576 |
| 88 | M35 | Mushito | Salvador | 20°18’ 15.04" | 103°10’ 47.80" | 1538 |
| 89 | M36 | Mushito | Chilchota | 19°50’ 33.78" | 102°6’ 33.43" | 1796 |
| 90 | M37 | Ratón tamaulipas | Tzizio | 19°35’ 3.08" | 100°55’ 13.99" | 1592 |
| 91 | M38 | Ratón tamaulipas | Arteaga | 18°21’ 40.01" | 102°16’ 55.00" | 909 |
| 92 | M39 | Ratón tamaulipas | Tepalcatepec | 19°11’ 6.01" | 102°49’ 45.57" | 366 |
| 93 | M40 | Ratón tamaulipas | Huetamo | 18°38’ 5.56" | 100°54’ 44.23" | 303 |
| 94 | M41 | Ratón tamaulipas | Tuzantla | 19°13’ 14.28" | 100°34’ 16.00" | 603 |
| 95 | M42 | Ratón tamaulipas | Ziracuaretiro | 19°24’ 41.60" | 101°54’ 19.58" | 1366 |
| 96 | M43 | Ratón tamaulipas | Benito Juarez | 19°18’ 49.73" | 100°25’ 46.97" | 1341 |
| 97 | M44 | Ratón tamaulipas | Chinicuila | 18°2’ 1.05" | 102°12’ 50.64" | 55 |
| 98 | M45 | Ratón tamaulipas | Tumbiscatio | 18°31’ 7.86" | 102°22’ 56.99" | 955 |
| 99 | M46 | Ratón tamaulipas | Coalcoman | 18°45’ 56.63" | 103°8’ 42.43" | 1260 |
| 100 | M47 | Ratón tamaulipas | Huacana | 18°58’ 6.94" | 101°48’ 38.59" | 509 |
| 101 | M48 | Tuxpeño | Churumuco | 19°3’ 48.96" | 102°21’ 14.05" | 302 |
| 102 | M49 | Tuxpeño | Aguililla | 18°43’ 53.78" | 102°46’ 51.64" | 912 |
| 103 | M50 | Tuxpeño | Coalcoman | 18°46’ 2.68" | 103°8’ 39.19" | 1257 |
| 104 | M51 | Tuxpeño | Tiquicheo | 18°54’ 3.98" | 100°44’ 39.38" | 404 |
| 105 | M52 | Tuxpeño | Tepalcatepec | 19°10’ 15.83" | 102°51’ 10.16" | 373 |
| 106 | M53 | Tuxpeño | Tepalcatepec | 19°10’ 4.51" | 102°50’ 39.39" | 357 |
| 107 | M54 | Vandeño | Turicaro | 19°34’ 10.17" | 101°56’ 4.08" | 2370 |
| 108 | M55 | Vandeño | San Lucas | 18°34’ 33.51" | 100°47’ 14.59" | 298 |
| 109 | M56 | Vandeño | San Lucas | 18°34’ 45.81" | 100°47’ 1.00" | 317 |
| 110 | M57 | Zamorano amarillo | Penjamillo | 20°4’ 26.01" | 101°56’ 16.43" | 1795 |
| 111 | M58 | Zamorano amarillo | Penjamillo | 20°4’ 25.64" | 101°56’ 18.32" | 1800 |
| 112 | M59 | Zamorano amarillo | Penjamillo | 20°4’ 22.27" | 101°56’ 19.08" | 1792 |
| 113 | M60 | Zamorano amarillo | Penjamillo | 20°4’ 25.92" | 101°56’ 21.25" | 1806 |
| 114 | M61 | Tabloncillo | San Juanito | 19°40’ 28.27" | 101°15’ 5.32" | 1895 |
| 115 | M62 | Tabloncillo | Tumbiscatio | 18°31’ 7.35" | 102°22’ 28.58" | 919 |
| 116 | M63 | Tabloncillo | Chinicuila | 18°1’ 53.26" | 102°12’ 49.82" | 34 |
| 117 | M64 | Tsiri charapiti | Patzcuaro | 19°30’ 42.45" | 101°35’ 26.32" | 2285 |
| 118 | M65 | Tsiri charapiti | Tingambato | 19°29’ 59.91" | 101°51’ 33.40" | 1945 |
| 119 | M66 | Elotes conicos | Tingambato | 19°30’ 19.65" | 101°51’ 38.13" | 1955 |
| 120 | M67 | Elotes conicos | Tingambato | 19°30’ 6.30" | 101°51’ 36.04" | 1949 |
| 121 | M68 | Elotes conicos | Tingambato | 19°29’ 33.01" | 101°50’ 58.55" | 1968 |
| 122 | M69 | Elotero sinaloa | Buenavista | 19°12’ 11.08" | 102°35’ 51.87" | 453 |
| 123 | M70 | Elotero sinaloa | Aguililla | 18°44’ 9.44" | 102°46’ 24.09" | 909 |
| 124 | M71 | Elotero sinaloa | Arteaga | 18°21’ 17.52" | 102°16’ 43.37" | 856 |
| 125 | M72 | Elotero sinaloa | Tacambaro | 19°13’ 25.94" | 101°27’ 20.50" | 1569 |
| 126 | M73 | Elotero sinaloa | Aquila | 18°36’ 3.48" | 103°29’ 48.02" | 273 |
| 127 | M74 | Elotero sinaloa | Coahayana | 18°51’ 1.77" | 103°37’ 12.58" | 46 |
| 128 | M75 | Elotero sinaloa | Chinicuila | 18°2’ 6.06" | 102°13’ 4.91" | 63 |
| 129 | M76 | Elotero sinaloa | Tamazula | 19°34’ 51.97" | 102°27’ 6.88" | 1367 |
| 130 | M77 | Elotero sinaloa | Tecatitlan | 19°21’ 48.78" | 103°1’ 6.03" | 801 |
| 131 | M78 | Elotero sinaloa | Jilotlan | 19°22’ 15.71" | 103°0’ 44.48" | 754 |
| 132 | M79 | Elotero sinaloa | Paramo | 19°22’ 43.12" | 102°1’ 38.35" | 1589 |
| 133 | M80 | Reventador | Aquila | 18°36’ 8.32" | 103°30’ 18.21" | 197 |
| 134 | M81 | Reventador | Aquila | 18°35’ 50.92" | 103°30’ 28.31" | 233 |
| 135 | M82 | Reventador | Aquila | 18°35’ 33.14" | 103°30’ 29.07" | 190 |
| 136 | M83 | Reventador | Aquila | 18°35’ 38.66" | 103°30’ 0.93" | 242 |
| 137 | M84 | Reventador | Aquila | 18°36’ 14.80" | 103°29’ 59.85" | 276 |
| 138 | M85 | Arrocillo | Aporo | 19°39’ 53.63" | 100°24’ 33.59" | 2292 |
| 139 | M86 | Arrocillo | Angangueo | 19°36’ 35.63" | 100°17’ 30.43" | 2616 |
| 140 | M87 | Arrocillo | Zitacuaro | 19°26’ 55.95" | 100°19’ 59.13" | 2132 |
| 141 | M88 | Arrocillo | Ocampo | 19°35’ 38.35" | 100°20’ 26.80" | 2320 |
| 142 | M89 | Arrocillo | Ocampo | 19°35’ 31.45" | 100°20’ 48.17" | 2304 |
| 143 | M90 | Perepecha | Ocampo | 19°35’ 14.77" | 100°20’ 43.80" | 2300 |
| 144 | M91 | Perepecha | Ocampo | 19°34’ 59.68" | 100°20’ 47.52" | 2287 |
| 145 | M92 | Dulce | Ocampo | 19°34’ 48.96" | 100°20’ 58.47" | 2275 |
| 146 | M93 | Dulce | Ocampo | 19°34’ 34.88" | 100°21’ 5.35" | 2278 |
| 147 | M94 | Dulce | Ocampo | 19°34’ 36.47" | 100°20’ 43.99" | 2292 |
| 148 | M95 | Dulce | Ocampo | 19°34’ 31.59" | 100°20’ 28.88" | 2326 |
| 149 | M96 | Dulce | Ocampo | 19°34’ 25.16" | 100°20’ 19.39" | 2340 |
| 150 | O1 | tuxpen | San Jose Chiltepec | 17°57’ 17.68" | 96°9’ 18.15" | 51 |
| 151 | O1 | tuxpen. . .olotil | San Jose Chiltepec | 17°57’ 14.59" | 96°9’ 30.96" | 53 |
| 152 | O3 | tuxpen. . .tepeci | Valle Nacional | 17°45’ 44.92" | 96°13’ 12.70" | 61 |
| 153 | O4 | tepeci. . .dzitba | Valle Nacional | 17°48’ 37.49" | 96°13’ 26.59" | 50 |
| 154 | O5 | tepeci | San Juan Lalana | 17°27’ 10.45" | 95°45’ 59.76" | 249 |
| 155 | O6 | olotil | San Juan Lalana | 17°27’ 0.35" | 95°46’ 2.01" | 208 |
| 156 | O7 | hibrido tuxpen | Santiago Yaveo | 17°19’ 49.25" | 95°41’ 57.85" | 339 |
| 157 | O8 | olotil 8 | Santiago Yaveo | 17°19’ 46.67" | 95°42’ 55.07" | 218 |
| 158 | O9 | tepeci. . .zapagr | San Juan Guichicovi | 16°59’ 22.77" | 95°1’ 15.84" | 108 |
| 159 | O10 | zapagr. . .olotil | San Juan Guichicovi | 16°58’ 40.64" | 95°1’ 5.03" | 121 |
| 160 | O11 | tepeci. . .tuxpen | San Juan Cotzocon | 17°9’ 36.23" | 95°46’ 40.41" | 1408 |
| 161 | O12 | tepeci | Santiago Yaveo | 17°19’ 47.81" | 95°40’ 9.59" | 339 |
| 162 | O13 | olotil. . .zapagr | Santiago Yaveo | 17°20’ 8.96" | 95°40’ 41.55" | 346 |
| 163 | O14 | chalou. . .bolita | Nochistlan | 17°28’ 12.90" | 97°16’ 23.45" | 2052 |
| 164 | O15 | chalou | Nochistlan | 17°27’ 40.46" | 97°16’ 2.89" | 2046 |
| 165 | O16 | bolita | Nochistlan | 17°26’ 55.85" | 97°14’ 51.77" | 2055 |
| 166 | O17 | bolita conico | Nochistlan | 17°31’ 19.09" | 97°16’ 44.99" | 2090 |
| 167 | O18 | connor. . .pepiti | Nochistlan | 17°30’ 32.38" | 97°16’ 46.06" | 2082 |
| 168 | O19 | bolita | San Andres Andua | 17°26’ 52.71" | 97°18’ 11.45" | 2055 |
| 169 | O20 | bolita | San Andres Andua | 17°26’ 9.44" | 97°17’ 36.60" | 2048 |
| 170 | O21 | bolita | San Andres Andua | 17°27’ 10.80" | 97°16’ 2.49" | 2039 |
| 171 | O22 | chalou | La Paz | 17°16’ 51.35" | 97°20’ 25.23" | 2221 |
| 172 | O23 | chalou | La Paz | 17°17’ 0.92" | 97°20’ 14.93" | 2178 |
| 173 | O24 | chalou. . .bolita | San Juan Diuxi | 17°17’ 6.84" | 97°22’ 31.82" | 2316 |
| 174 | O25 | chalou 9 | San Juan Diuxi | 17°17’ 0.14" | 97°22’ 49.39" | 2472 |
| 175 | O26 | bolita | San Pedro Topiltepec | 16°39’ 46.52" | 96°17’ 16.90" | 922 |
| 176 | O27 | chalou | Santo Domingo Yanhuitlan | 17°31’ 20.43" | 97°20’ 13.34" | 2143 |
| 177 | O28 | bolita. . .chalou | Santo Domingo Yanhuitlan | 17°32’ 16.36" | 97°20’ 53.48" | 2182 |
| 178 | O29 | tepeci. . .tuxpen | Pinotepa de Don Luis | 16°25’ 23.51" | 97°59’ 11.91" | 435 |
| 179 | O30 | tepeci. . .olitill | San Juan Colorado | 16°27’ 22.17" | 97°57’ 29.96" | 447 |
| 180 | O31 | tuxpen. . .olotill | Pinotepa de Don Luis | 16°25’ 37.07" | 97°57’ 43.71" | 427 |
| 181 | O32 | olotil 9 | San Pedro Jicayan | 16°26’ 51.50" | 98°1’ 26.79" | 336 |
| 182 | O33 | tepeci. . .olotill | San Pedro Jicayan | 16°27’ 15.94" | 98°1’ 19.89" | 348 |
| 183 | O34 | tepeci. . .tuxpen | San Pedro Jicayan | 16°26’ 36.04" | 98°1’ 8.91" | 287 |
| 184 | O35 | olotil 7 | San Pedro Jicayan | 16°27’ 40.02" | 98°0’ 45.03" | 394 |
| 185 | O36 | olotil 8 | San Miguel Tlacamama | 16°25’ 10.33" | 98°3’ 36.81" | 276 |
| 186 | O37 | tuxpen | San Miguel Tlacamama | 16°24’ 38.92" | 98°3’ 35.07" | 306 |
| 187 | O38 | tuxpen. . .fasciado | Santiago Pinotepa Nacional | 16°11’ 1.35" | 97°58’ 6.80" | 16 |
| 188 | O39 | tuxpen. . .olotill | Santa Maria Tonameca | 15°44’ 38.05" | 96°33’ 9.85" | 31 |
| 189 | O40 | olotill 7 | Santa Maria Tonameca | 15°44’ 30.90" | 96°33’ 1.71" | 28 |
| 190 | O41 | tuxpen. . .tepeci | Santa Maria Tonameca | 15°44’ 58.27" | 96°32’ 27.13" | 32 |
| 191 | O42 | tuxpen. . .olotill | San Pedro Mixtepec | 15°59’ 37.02" | 97°5’ 30.37" | 310 |
| 192 | O43 | olotil 9 | Pinotepa de Don Luis | 16°25’ 11.27" | 97°58’ 31.62" | 442 |
| 193 | O44 | tuxpeño | San Meteo Sindihui | 16°59’ 58.07" | 97°20’ 55.73" | 1484 |
| 194 | O45 | tuxpeño | San Meteo Sindihui | 17°0’ 26.56" | 97°21’ 8.24" | 1461 |
| 195 | O46 | tuxpeño | San Meteo Sindihui | 17°0’ 1.51" | 97°21’ 9.48" | 1471 |
| 196 | P1 | Cacahuacintle | Chignahuapan | 19°50’ 44.87" | 98°1’ 25.64" | 2268 |
| 197 | P2 | Conico Amarillo | Zacatlan | 19°56’ 9.52" | 97°56’ 39.16" | 2032 |
| 198 | P3 | Arrocillo | San Nicolas Buenos Aires | 18°29’ 43.52" | 97°25’ 42.18" | 1687 |
| 199 | P4 | Conico Amarillo | San Martin Texmelucan | 19°17’ 42.29" | 98°26’ 24.18" | 2263 |
| 200 | P5 | Palomero Blanco | Tetela de Ocampo | 19°48’ 46.58" | 97°48’ 16.33" | 1759 |
| 201 | P6 | Chalqueño | Aljojuca | 19°5’ 55.26" | 97°32’ 20.83" | 2444 |
| 202 | P7 | Elotes Conicos | Tlachichuca | 19°6’ 46.89" | 97°24’ 39.80" | 2639 |
| 203 | P8 | Conico Blanco | San Juan Tianguismanalco | 18°56’ 5.44" | 98°27’ 38.26" | 1951 |
| 204 | P9 | Chalqueño | Tepatlaxco | 19°4’ 16.79" | 97°58’ 11.54" | 2370 |
| 205 | P10 | Elotes Conicos | Chalchicomula de Sesma | 18°57’ 2.17" | 98°12’ 44.96" | 2071 |
| 206 | P11 | Cacahuacintle | Tlachichuca | 19°7’ 35.00" | 97°25’ 18.39" | 2588 |
| 207 | P12 | Olotillo | Xicotepec | 18°56’ 53.77" | 98°15’ 36.60" | 2098 |
| 208 | P13 | Tuxpeño | Francisco Z. Mena | 18°32’ 9.70" | 98°30’ 10.43" | 1210 |
| 209 | P14 | Amilaceo | Tetela de Ocampo | 19°49’ 24.79" | 97°48’ 41.60" | 1698 |
| 210 | P15 | Conico Amarillo | Tepatlaxco | 19°4’ 49.67" | 97°57’ 38.96" | 2390 |
| 211 | P16 | Pepitilla Morado | Atlixco | 18°54’ 4.80" | 98°25’ 49.97" | 1826 |
| 212 | P17 | Ancho | Atlixco | 18°53’ 58.65" | 98°27’ 6.57" | 1862 |
| 213 | P18 | Ancho | Cohuecan | 18°46’ 56.70" | 98°42’ 58.81" | 1706 |
| 214 | P19 | Vandeño | Albino Zertuche | 18°1’ 3.30" | 98°32’ 3.45" | 1313 |
| 215 | P20 | Pepitilla Blanco | Tochimilco | 18°53’ 7.39" | 98°34’ 34.34" | 2075 |
DNA extraction, PCR amplification and detection of transgenes in vegetal material
Seeds from each collected variety were planted and germinated to obtain foliar tissue for GMO-DNA analyses. Polystyrene trays were filled with sterile forest soil and substrate (peat-moos) in a 6:4 ratio. After, a group of 10 seeds of each maize variety were planted. At 14 days after plantlet emergence, leaf tissue from 10 germinated seeds was cutting in mass and stored at 4 °C. For DNA isolation was used the method proposed by Graham et al. (1995). DNA integrity was determined by electrophoresis in 1.5% agarose gel and DNA quality was estimated in an Epoch Microplate Spectrophotometer with the Gen5 1.11 software. A Polymerase Chain Reaction (PCR) was performed with each DNA sample composite to amplify eight accompanying sequences (Table 2) and nine transgenic events (Table 3), in this case, specific primers and specific amplification temperatures for each of these sequences were used. Most of the published studies analyzed one or a few transgenic sequences and, in some cases, only a few samples of maize varieties and most of the studies were concentrated in Oaxaca (Mercer and Wainwright 2008). The PCR amplified segments were visualized using agarose gel electrophoresis (1.5%). The molecular markers of 50bp and 100bp DNA ladder (Invitrogen) were used as reference for determination of amplified segments size. In addition, the different amplified segments were sequenced to corroborate the identity of the amplified sequence.
Table 2 Target sequence, primer sequence, sequence size, and annealing temperature used during PCR, for detection of accompanying transgenic sequences.
| Target sequence | Primer sequence | Sequence size (bp) | Annealing temperature (°C) |
|---|---|---|---|
| ubi [25] | F-5’-gctaacttgccagtgtttctctttgg-3’ | 220 | 55 |
| R-5’-ggctggcattatctactcgaaacaag-3’ | |||
| 35 s [24] | F-5’-gctcctacaaatgccatca-3’ | 238 | 60 |
| R-5’-actgcgtgttagggtgatag-3’ | |||
| bar [26] | F-5’-gcacagggcttcaagagcgtggtc-3’ | 177 | 55 |
| R-5’-gggcggtaccggcaggctgaa-3’ | |||
| ntpII [26] | F-5’-gaggctattcggctatgact-3’ | 271 | 64 |
| R-5’-aaggtgagatgacaggagat-3’ | |||
| uidA [27] | F-5’-ggtgggaaagcgcgttacaag-3’ | 150 | 55 |
| R-5’-accgccttcgttgcgcatttg-3’ | |||
| luc [28] | F-5’-cgccaaaaacataaagaaaggc-3’ | 450 | 64 |
| R-5’-tgtccctatcgaaggactctgg-3’ | |||
| ocs [27] | F-5’-ctcgagctgctttaatgagatatgcg-3’ | 120 | 55 |
| R-5’-tctagactgctgagcctcgacatgttg-3’ | |||
| nos [26] | F-5’-gaatcctgttgccggtcttg-3’ | 125 | 57 |
| R-5’-gcgggactctaatcataaaaacc-3’ |
Table 3 Target gene, primer sequence, sequence size, and annealing temperature used during PCR for detection of transgenic events.
| Gene | Primer sequence | Sequence size (bp) | Annealing temperature (°C) |
|---|---|---|---|
| cry 3a | F-5’-acatgcatgcattaactagaaagtaaagaagtag-3’ | 479 | 62.6 |
| R-5’-acatgcatgcaagcttacagagaaatacacgaggg-3’ | |||
| cry2a | F-5’-gctctagaataggaggaaaagattttatgctaaaa-3’ | 850 | 62.6 |
| R-5’-acgcgtcgacaaatatctagttttatattaa-3’ | |||
| cry 1e | F-5’-ggatcccatatggagatagtg-3’ | 756 | 64 |
| R-5’-cgcggatcctatctagaatcgtaatt-3’ | |||
| ec | F-5’-ccagtctgttgacctggttgt-3’ | 239 | 64.7 |
| R-5’-tttctgcagatgtcaacgtattctatacc-3’ | |||
| cry11a | F-5’-acatgcatgcagtcatgttagcacaagagga-3’ | 850 | 62.6 |
| R-5’-acatgcatgctttaggtctttaaaaattaga-3’ | |||
| Accasa | F-5’taggactggtaccgtaaagcagagtaacacaaggtcag-3’ | 514 | 64.7 |
| R-taggactctcgagagtctttcggaacctcacaccataagg-3’ | |||
| Als | F-5’-gggttacgcacgcgccaccgg-3’ | 397 | 64 |
| R-5’-ggctgatcccagtcaggtatc-3’ | |||
| cry 1ab | F-5’-accatcaacagccgctacaacgacc-3’ | 184 | 70 |
| R-5’-tggggaacaggctcacgatgtccag-3’ | |||
| Epsps | F-5’-tggcgcccaaagcttgcatggc-3’ | 356 | 62 |
| R-5’-ccccaagttcctaaatcttcaagt-3’ |
Determination the level of introgression of transgenes into maize varieties
The level of introgression of transgenic event and accompanying sequence into local maize varieties which were planted as not biotech crops was determined in percent, in respect to varieties with presence of GMO into its genome vs total number of analyzed maize varieties in each Mexican State.
Results
Collection of maize seed
Each collection site of maize varieties was georeferenced on a map (Figure 1). From 215 maize seed samples, 24.65% were from Jalisco, 44.65% from Michoacán, 21.39% from Oaxaca and 9.3% from Puebla.
Detection of transgenic sequences in plant tissue
In this study, it was only detected the presence of the cry1Ab transgenic event in all the maize varieties collected in the Mexican Central Region, and the ntpII accompanying sequence only was detected in Michoacan and Jalisco; which suggest that local maize varieties from these Mexican States are contaminated with transgene insert.
Estimate of maize contamination frequencies
Only the number of varieties positive for the transgenes cry1Ab and ntpII presence were included in the statistical analysis. In this analysis about frequency of these transgenic sequences present in the maize varieties were detected highly significant differences (P < = 0.01) (Table 4), the frequency rate for the cry1Ab gene was 41%, while the frequency rate for the ntpII sequence was 9.7%, in this analysis Q value was 56.2661 which was strongly significant and indicated that both frequencies are different. One explanation may be that during transformation of plants with the cry1Ab gene, different marker genes are utilized which may be different to ntpII.
Table 4 Statistical values of the categorical analysis in SxR tables for the presence of transgenic sequences in maize varieties in Mexico.
| Statistic | df | Value | Probability |
|---|---|---|---|
| Chi-square | 1 | 56.3966 | <0.0001 |
| Likelihood Ratio chi-square | 1 | 59.7029 | <0.0001 |
| Continuity Adj. chi-square | 1 | 54.7501 | <0.0001 |
| Mantel-Haenszel Chi-square | 1 | 56.2661 | <0.0001 |
| Phi Coefficient | -0.3613 | ||
| Contingency Coefficient | 0.3398 | ||
| Cramer’s V | -0.3613 |
The frequency of transgenic events presents in to the maize varieties from Puebla, Oaxaca, Jalisco and Michoacán, are shown in Table 5. The present study focused on detect transgenic events and accompanying sequences into maize varieties by PCR and using seeds collected of local maize varieties from Puebla, Jalisco, Michoacán and Oaxaca States, allowed to detecting the presence of the cry1Ab transgenic gene and the presence of the ntpII accompanying sequence in the maize varieties (Table 5). The localities with presence of the cry1Ab transgenic event and accompanying sequence ntpII in the maize populations are described in Table 6.
Table 5 Frequency (%) of transgenic events transferred to the maize varieties collected in Puebla, Oaxaca, Jalisco and Michoacán States.
| Transgenic sequence | non- introgressed Genotypes | Percent | Introgressed Genotypes | Percent | Total |
|---|---|---|---|---|---|
| cry1Ab | 127 | 58.80 | 89 | 41.20 | 216 |
| ntpII | 195 | 90.28 | 21 | 9.72 | 216 |
Table 6 Localities with presence transgenic event (cry1Ab) and accompanying sequence (ntpII) in maize varieties collected from Jalisco, Michoacán, Oaxaca and Puebla States.
| Varieties corn | Varieties corn | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Key | Name | Locality | Cry1Ab | ntpII | Key | Name | Locality | Cry1Ab | ntpII |
| J11 | Celaya 2010(15) | San Juan de los Lagos | 1 | 0 | M91 | Perepecha | Ocampo | 1 | 0 |
| J13 | Celaya 2010(17) | Lagos de Moreno | 1 | 1 | M92 | Dulce | Ocampo | 1 | 0 |
| J14 | Celaya 2010(18) | Lagos de Moreno | 1 | 1 | M93 | Dulce | Ocampo | 1 | 0 |
| J21 | Celaya 2010(26) | Mazamitla | 1 | 0 | M94 | Dulce | Ocampo | 1 | 0 |
| J24 | Celaya 2010(29) | Tala | 1 | 0 | M95 | Dulce | Ocampo | 1 | 0 |
| J28 | Celaya 2010(35) | Sayula | 1 | 0 | M96 | Dulce | Ocampo | 1 | 0 |
| J29 | Celaya 2010(37) | Sayula | 1 | 0 | O1 | Tuxpen | San Jose Chitepec | 1 | 0 |
| J30 | Celaya 2010(38) | Gomez Farias | 1 | 0 | O10 | zapagro-olotillo | San Juan Guichicovi | 1 | 0 |
| M13 | Celaya | Huaniqueo | 1 | 1 | O11 | tepeci..tuxpen | San Juan Cotzocon | 1 | 0 |
| M14 | Chalqueño | Epitacio Huerta | 1 | 1 | O12 | tepeci | Santiago Yaveo | 1 | 0 |
| M15 | Chalqueño | Angangueo | 1 | 1 | O13 | olotil. . .zapagr | Santiago Yaveo | 1 | 0 |
| M16 | Chalqueño | Ocampo | 1 | 1 | O15 | Chalou | Nochistlan | 1 | 0 |
| M17 | Conico | Morelos | 1 | 1 | O16 | Bolita | Nochistlan | 1 | 0 |
| M18 | Conico | Morelos | 1 | 1 | O17 | Bolita conico | Nochistlan | 1 | 0 |
| M19 | Conico | Morelos | 1 | 0 | O18 | connor. . .pepiti | Nochistlan | 1 | 0 |
| M21 | Conico | Patzcuaro | 1 | 1 | O19 | Bolita | San Andres Andua | 1 | 0 |
| M40 | Ratontamaulipas | Huetamo | 1 | 1 | O2 | Tuxpen. . .olotil | San Juan Chitepec | 1 | 0 |
| M48 | Tuxpeño | Churumuco | 1 | 0 | O20 | Bolita | San Juan Andua | 1 | 0 |
| M49 | Tuxpeño | Aguililla | 1 | 0 | O26 | Bolita | San Pedro Topiltepec | 1 | 0 |
| M51 | Tuxpeño | Tiquicheo | 1 | 0 | O27 | Chalou | Santo Domingo Yanhuitlan | 1 | 0 |
| M52 | Tuxpeño1 | 0Tepalcatepec | 1 | 0 | O29 | tepeci. . .tuxpen | Pinotepa de Don Luis | 1 | 0 |
| M53 | Tuxpeño | Tepalcatepec | 1 | 0 | O4 | tepeci. . .dzitba | Valle Nacional | 1 | 0 |
| M66 | Elotes Conicos | Tingambato | 1 | 1 | O41 | Tuxpen. . .tepeci | Santa maria Tonameca | 1 | 0 |
| M67 | Elotes Conicos | Tingambato | 1 | 0 | O42 | Tuxpen. . .olotil | San Pedro Mixtepec | 1 | 0 |
| M68 | Elotes Conicos | Tingambato | 1 | 1 | O43 | Olotil9 | Pinotepa de Don Luis | 1 | 0 |
| M69 | Elotero Sinaloa | Buenavista | 1 | 1 | O45 | Tuxpeño | San Meteo Sindihui | 1 | 0 |
| M70 | Elotero Sinaloa | Agulilla | 1 | 0 | O46 | Tuxpeño | San Meteo Sindihui | 1 | 0 |
| M72 | Elotero Sinaloa | Tacambaro | 1 | 0 | O7 | Hibrid Tuxpen | Santiago Yaveo | 1 | 0 |
| M74 | Elotero Sinaloa | Coahayana | 1 | 0 | O8 | Olotil 8 | Santiago Yaveo | 1 | 0 |
| M75 | Elotero Sinaloa | Chinicuila | 1 | 0 | O9 | Tepeci. . .zapagr | San Juan Guichicovi | 1 | 0 |
| M76 | Elotero Sinaloa | Tamazula | 1 | 1 | P1 | Cacahuacintle | Chingnahuapan | 1 | 0 |
| M77 | Elotero Sinaloa | Tecatitlan | 1 | 1 | P2 | Conico Amarillo | Zacatla | 1 | 0 |
| M78 | Elotero Sinaloa | Jilotlan | 1 | 1 | P3 | Arrocillo | San Nicolas Buenos Aires | 1 | 0 |
| M79 | Elotero Sinaloa | Paramo | 1 | 1 | P4 | Conico Amarillo | San Martin Texxmelucan | 1 | 0 |
| M80 | Reventador | Aquila | 1 | 1 | P6 | Chalqueño | Aljojuca | 1 | 0 |
| M81 | Reventador | Aquila | 1 | 0 | P7 | Elotes Conicos | Tlachichuca | 1 | 0 |
| M82 | Reventador | Aquila | 1 | 0 | P8 | Conico Blanco | San Juan Tiangusmanalco | 1 | 0 |
| M83 | Reventador | Aquila | 1 | 0 | P10 | Elotes Conicos | Chalchicomula de Sesma | 1 | 0 |
| M84 | Reventador | Aquila | 1 | 0 | P12 | Olotillo | Xicotepec | 1 | 0 |
| M85 | Arrocillo | Aporo | 1 | 0 | P14 | Amilaceo | Tetela de Ocampo | 1 | 0 |
| M86 | Arrocillo | Zitacuaro | 1 | 0 | P18 | Ancho | Cohuecan | 1 | 0 |
| M88 | Arrocillo | Ocampo | 1 | 0 | P19 | Vandeño | Albino Zertuche | 1 | 0 |
| M89 | Arrocillo | Ocampo | 1 | 0 | P20 | Pepetilla Blanco | Tochimilco | 1 | 0 |
| M90 | Perepecha | Ocampo | 1 | 1 | |||||
Introgression of transgenes into maize varieties
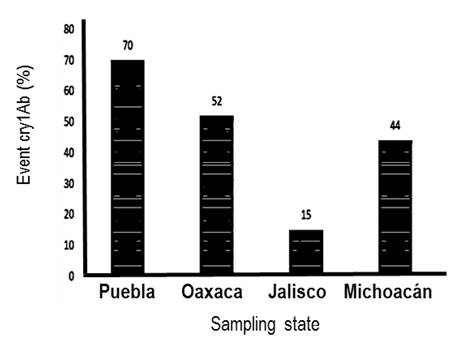
According to the presence of cry1Ab into the genome of the sampled maize varieties from these four Mexican states, it was observed that maize varieties grown as non-biotech crops from Puebla State have the highest levels (70%) of contamination o introgression of transgenes inserts into their genomes, followed by maize variety samples from Oaxaca with 52.17%, Michoacán maize samples with 44.79% and Jalisco maize samples with 15.09%, respectively. While, the presence of accompanying sequence ntpII was only detected in the Michoacán and Jalisco maize samples in 89.47 and 10.52%, respectively (Figure 2).
Discussion
Collection of maize seed
Although some studies have confirmed the contamination of native maize by transgenic events, these studies considered a low number of samples, for example, De-Ita (2012) indicates the use of samples from 22 localities, while The National Commission for the Knowledge and Use of Biodiversity (Conabio) confirmed the presence of transgenes from 3 to 10% in 15 locations. Another study for the determination of transgenes carried out by Serratos-Hernández et al. 2007 is based on a sample of 25 communities. The present study considered a greater number of localities (215) from 4 Mexican States, which allows to have a better perspective of the contamination of native maize by transgenic segments.
Detection of transgenic sequences in plant tissue
These results confirm the introgression of transgenes into maize varieties as indicated by (Quist and Chapela 2001, Cleveland et al. 2005, Serratos-Hernández et al. 2007, Mercer and Wainwright 2008, Piñeyro et al. 2009, Orozco-Ramírez et al. 2016, Rendón-Aguilar et al 2019) who reported the presence of transgenic DNA in native varieties of maize grown in remote mountains of Oaxaca, this place is part of the center of origin and diversification of the crop in Mexico.
Estimate of maize contamination frequencies
The high detection rate of the cry1Ab transgenic event in the maize varieties populations implies that protective measures must be taken in the short term to produce maize varieties seeds free of these transgenic segments. This detection is controversial because in Mexico, it is not allowed to grow commercially maize transgenic cultivars, only at an experimental level (García and Toscana 2016, Ortega-Villegas et al. 2018, Lopez-Hernandez 2020), precisely, for preservation of biodiversity of the native maize, because Mexico is considered a center of origin and diversity of this commodity (Lohn et al. 2021).
Introgression of transgenes into maize varieties
This level of introgression of transgenes into the tested maize varieties could be considered very high, as it is determined in a specific way, what was found for each state (Figure 2), and assuming that the states with the lowest experimental seeding (Puebla and Oaxaca) are those with the greater genetic contamination, having thus that 70% of the varieties collected in Puebla state presented residues of the cry1Ab gene; while, 52% of the maize collected in Oaxaca were positive for the cry1Ab gene. On the other hand, in these states, not varieties were detected contaminated with accompanying sequences.
The cry1Ab gene was detected in 15% of the maize varieties collected in Jalisco, and 4 out of 53 samples collected were found to be introgressed with the accompanying ntpII sequence. While 44% of the varieties collected in Michoacán were observed to have the cry1Ab gene, and 17.7% of the samples collected in this state showed the accompanying ntpII sequence. The presence of transgenic events in maize varieties could have an cultural, economic, and social impact, on continuity of indigenous and peasant peoples as refer (Sánchez and Romero 2018, Ibarrola-Rivas et al. 2020), who point out the importance of this area on maize conservation, it is in this territory where the milpa takes place, and at the same time, the milpa is part of the territory; and with the cultural and natural characteristics of the territory, particular milpa are configured. Therefore, the contamination of maize or its substitution by transgenic varieties is not limited to changes in maize as a source of food, but also implies negative repercussions for farmers in the milpa and in the territory, increasing the risk to their cultural continuity. Milpa is defined as agroecosystem conformed by polyculture, which is a dynamic space of genetic resources (Ortiz et al. 2014, González and Reyes 2014, Fonteyne et al. 2023). CCA (2004) mentioned that in Mexico, maize is not only a commercial commodity, it is the base of Mexican diet and constitutes an integral expression of the relation between nature and culture, from this relation depends the subsistence of a great part of the Mexican rural population, and through this relation, the social tissue and people of these communities and diversity conservation are fortified.
Milpa is not only the space where maize, and other plant species are sown, but it is also a space for linguistic, cultural, symbolic, spiritual, social, and economical activities and food (Agapito-Tenfen et al. 2017, Ibarrola-Rivas et al. 2020), is more than a farming technique, it is an agroecosystem in which farmers of Mesoamerican origin cultivate dozens of comestible and medicinal herbs, along with fruit and timber trees. For these communities, the most important thing is that the diversity of the maize germplasm can be lost. Year after year, the farmers keep the best seeds for planting in the following season, passing the knowledge of selection of them, the preparation of the land and the accumulation of information on the weather, among other elements, to the next generation (Sánchez and Romero 2018). Maize varieties genetic diversity offers specialized genotypes which have demonstrated capacity of adaptation to different environments, pest and disease resistance, and satisfy divers demands for culinary, artisanal or industrial uses; this diversity is a key factor for the Mexican food security (WHO 2015).
It is considered that maize is a continuous process of domestication, since it depends completely on the farmer, who through selection has favored the survival, and reproduction of phenotypes that have advantageous characteristics to be used mainly as food for humans, and to date represent a total of 64 native maize races reported for Mexico (Santillán-Fernández et al. 2021). It is also convenient to clarify that natural selection operates during the process of domestication. The domestication process results in Mexico being a center of maize genetic diversity (Santillán-Fernández et al. 2021), and is the Mexicans’ responsibility to preserve this great maize genetic diversity, in addition to conducting selection programs to gather more, and better characters in the plants that will serve as progenitors to the next generation. Agricultural and cultural practices have been very important during the whole process of maize domestication, since each variety have adapted to the specific environment that includes the cultivation method (Guzzon et al. 2021).
Maize is currently important in the diet of Mexicans and for many of them, it is their main source of protein and energy, which can be considered as cheap diet (Erenstein et al. 2022); with an average consumption of 350 g per capita per day in 600 different presentations of maize. Therefore, it has been pointed out that maize requires a special protection regime for genetically modified organisms that can negatively modify the nutritional qualities of this cereal (Brookes and Barfoot, 2016). The genetic contamination by transgenes of the natural ecosystems of Mexico has been documented. Since 2001, there have been isolated reports that indicate the unintentional presence of GM maize (Quist and Chapela 2001, Serratos-Hernández et al. 2007, Mercer and Wainwright 2008, Piñeyro et al. 2009) and there is only low work in Mexico in which the absence of GMOs was reported in maize (Cleveland et al. 2005, Rendón-Aguilar et al 2019). Most of the published studies analyzed one or a few transgenic sequences and, in some cases, only a few samples of maize varieties and most of the studies were concentrated in Oaxaca (Mercer and Wainwright 2008). On the other hand, there are reports that maize shipments imported for consumption have a large percentage of transgenic residues, in addition, the grain is viable. From 29 imported shipments of maize for consumption were analyzed for presence of transgenic residues, all the samples presented at least one transgenic sequence, either an event or an accompanying sequence (Carvajal et al. 2017). So, there is a risk that grains fall during transport, germinate on the sides of roads or railroads, or that farmers take these grains as seed and when they are planted in regions with high genetic diversity of maize, pollen can transfer transgenic genes to the native maize. It is proposed that the information obtained in this work be integrated into the National Program for the Monitoring of Genetically Modified Organisms, which will allow a diagnosis of the current situation in Mexico on the introgression of transgenes to the native maize and will serve as the basis for taking the actions necessary in matters of Biosafety.
Conclusions
The present study reports presence of transgenic events (cry1Ab), and sequences associated with the gene (ntpII) into maize varieties from Puebla, Michoacán, Oaxaca and Jalisco states in about 45% of the sampled localities. Corrective measures must be taken to prevent the contamination of Mexican maize varieties, and to train the varieties producers to clean the out-of-type phenotypes that could be sources of contamination of transgenic genes within the contaminated maize varieties with transgenes.











 nueva página del texto (beta)
nueva página del texto (beta)