INTRODUCTION
Antioxidant compounds of natural origin, such as phenols and flavonoids have received great relevance in research in last decades and can play an important role in reducing of several chronic degenerative diseases (Cavalaro et al. 2019). Propolis is becoming a good source of these types of compounds and this natural resinous material collected by honeybees from leaf and flower buds of many species of trees, has been used worldwide in traditional medicine for centuries (Pellati et al. 2011). In southeastern of México, Yucatán is well known for its propolis, produced by Apis mellifera, which collect resins mainly from native trees, as such as Piscidia piscipula and Bursera simaruba (Góngora 2006).
Since propolis is a natural product with several therapeutic and pharmacological properties, it has attracted interest of researchers in recent years. Some of therapeutic properties of propolis are well known, as such as its antiseptic, antitumoral (Froza et al. 2017), antioxidant (Kasiotis et al. 2017), and immunomodulatory activities (Bufalo et al. 2013), but they depend on the composition of propolis.
The bioactive compounds content of propolis varies according to the geographic region, environmental conditions, climate and collecting season (López et al. 2014). Although some studies with phenolic compounds have been carried out on anti-diabetic (Li et al. 2016) and anti-hypertensive activities, there are no in vitro studies about the effectiveness of bioactive compounds of propolis extracts (PEE) on inhibition of α-amylase, α-glucosidase and ACE-I enzymes in México. The inhibition of these enzymes is related to the possible antidiabetic and antihypertensive effect of the PEE.
In Yucatán, there are some reports in literature on the therapeutic properties of propolis (Herrera-López et al. 2020). However, there are no reports on the presence and comparative data on phenols, flavonoids, and pharmacological activities among samples of propolis gathered at different localities. Thus, the aim of the current study was to assess bioactive compounds and therapeutic activities of propolis of different locations, due that their comparative evaluation becomes important because the economic potential of propolis as a bee product has not been recognized in the region and in the country. Knowledge of the chemical composition and therapeutic properties will establish the importance of a product from honeybees, which until now has been little used by beekeepers.
MATERIALS AND METHODS
Propolis samples
Samples of 100 g of raw propolis were obtained of three apiaries of different locality from Yucatán (México); Maxcanú (west of the state) coordinates 20° 35’ latitude (North) and 90° 0’ longitude (West), Tizimín (east) coordinates 20° 57’ latitude (North) and 87° 31’ longitude (West), and Huhí (center) coordinates 20° 38’ latitude (North) and 89° 15’ longitude (West). The three localities were selected according to the presence of apiaries and number of hives of Apis mellifera. Samples were collected of thirteen hives using the traditional scraping method with stainless steel spatulas, the sample stuck in the lid of each hive box was removed and stored in a glass container for its transfer. Samples of raw propolis were cleaned removing pieces of wood, small stones, and parts of insects. These samples were maintained in freezing at 0 °C for further study and analysis.
Chemicals reagents
Catechin, 2,2-Diphenyl-1-picrylhydrazyl (DPPH), Folin-Ciocalteu, ferric chloride, 2,4,6-Tri(2-pyridyl)-s-triazine(TPTZ), acetic acid (glacial), aluminum chloride anhydrous, gallic acid, ethanol, sodium carbonate and phenols and flavonoids used as standards compounds were reagents analytical grade and purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Propolis ethanolic extract (PEE) preparation
Raw propolis (6 g) was mixed in 95% (v/v) ethanol (20 mL) and stirred every day for twenty-eight days at 25 °C. The extract solution was filtered and dried in a vacuum rotary evaporator (40 °C). The dry extract was lyophilized and preserved at -20 °C until further study and analysis. The extract of each sample was made in triplicate. All analyses were carried out in triplicate.
Phenolic and flavonoids compounds by HPLC
An aliquot of the PEE was dissolved with HPLC grade methanol (20 mg mL-1), the solution was filtered (0.45 μm Teknokroma filters), 20 𝜇L of the filtrate was injected into an Agilent HPLC-1220 using a Nucleosil C18 column (250 x 4.6 mm, 5 𝜇m); and detected at 280 nm. The mobile phase consisted of 1% formic acid in water (Phase A) (98%) and acetonitrile (Phase B) (2%) a flow of 0.5 mL min-1 was used. The separation was performed with linear gradient elution from 2 to 100% of phase B, from 0 to 70 min (Yahia et al. 2011). Identification was performed by comparison of retention times of injection of known compound standards (50 ppm).
Total phenols and flavonoids content
An aliquot of 100 𝜇L of PEE (10 mg mL-1) or each solution of standard (gallic acid, GA), was mixed with 500 𝜇L of Folin-Ciocalteu reagent, shaken for 30 s and left to stand for 2 min. Then, 400 𝜇L of Na2CO3 (75 mg mL-1) were added, incubated for 15 min at 50 °C and allowed to cool at room temperature. The absorbance was measured at 760 nm and expressed as gallic acid equivalent (GAE), (mg GAE g-1 of dry extract) (Brat et al. 2006) with a calibration curve of GA (20 -100 𝜇g mL-1). Total flavonoids content was determined using a colorimetric assay (Lee et al. 2003) using catechin as standard. An aliquot of 250 𝜇L of PEE (10 mg mL-1) or each standard solution was mixed with 250 𝜇L of 5% sodium nitrite; after 5 min, 125 𝜇L of 10% aqueous solution of aluminum chloride (AlCl3·6H30) was added. Subsequently, 124 𝜇L of sodium hydroxide (1M) was added 6 min after. The absorbance was measured at 490 nm. Total flavonoids content was expressed as catechin equivalent (CE), (mg CE/g of dry extract) (Brat et al. 2006) with a calibration curve (160 - 640 𝜇g mL-1).
DPPH* free radical-scavenging assay
With modifications DPPH* method was used (Shimada et al. 1992), PEE (100 𝜇L) (10 mg mL-1) was added to 1.0 mL of DPPH* 0.1 mM in 95% ethanol. The mixture was shaken for 30 min at room temperature and absorbance was measured at 517 nm. Ethanol was utilized as negative control and gallic acid (10 mg mL-1) as positive control. Antioxidant activity was expressed as the percentage inhibition of DPPH* free radical scavenging (% of DPPH*) calculated using the equation:
Where AC is the absorbance of DPPH* solution in absence of a sample added and AE is the absorbance of DPPH* in the presence of either the sample or standard after 30 min of reaction.
ABTS free radical-scavenging assay
The ABTS •+ radical cation method was used, reacting 2,2’-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid (ABTS) with potassium persulfate (Pukalskas et al. 2002). The ABTS •+ radical cation was produced reacting 10 mL ABTS stock solution (2 mM) with 40 𝜇L K2S4O8 solution 70 mM and allowing the mixture to stand in darkness at room temperature for 16-17 h before use. An aliquot of this ABTS solution was diluted with PBS (phosphate buffered saline pH 7.3) solution adjusting its absorbance at 0.800 ± 0.030 AU at 734 nm. For the analysis, to 990 𝜇L of this ABTS •+ solution, 10 𝜇L of 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) standard solution of different concentrations (0.5-3.5 mM) in PBS was added, then absorbance was read 6 min after mixing. Percentage decrease in absorbance at 734 nm was calculated and plotted as a function of Trolox solution concentration. Similarly, with the PEE extract a plot of percentage decrease in absorbance at 734 nm was obtained by reacting solutions of different concentration of PEE with ABTS •+ solution. In this method the total antioxidant activity was expressed as the Trolox Equivalent Antioxidant Capacity (TEAC) (mM Trolox/g dry PEE) which was calculated based on slopes of curves obtained by reaction different concentrations of PEE and Trolox (reference antioxidant compound). To calculate TEAC of PEE, the slope of the plot of the percentage inhibition of absorbance vs PEE concentration was divided by the slope of the Trolox plot (percentage inhibition of absorbance vs Trolox concentration). This produce the TEAC at the specific time. TEAC is one of the assays widely used to evaluate antioxidant activity of several types of compounds. This method was first developed as a simple and convenient method to determination of total antioxidant capacity (Miller et al. 1993).
ACE-I (angiotensin converting enzyme) inhibitory activity
With some modifications from Hayakari et al. (1978). Hippuryl-L-histidyl-L-leucine (HHL) is hydrolyzed by ACE to yield hippuric acid and histidyl-leucine. This method relies on the colorimetric reaction of hippuric acid with 2,4,6-trichloro-s-triazine (TT) in a solution (0.5 mL) containing 40 𝜇mol potassium phosphate buffer (pH 8.3), 300 𝜇mol sodium chloride, 40 𝜇mol 3% HHL in potassium phosphate buffer (pH 8.3), and 100 mU/ml ACE-I. The mixture was incubated at 37 °C for 45 min and the reaction was terminated by adding TT (3% v/v) in dioxane and 3 mL of 0.2 M potassium phosphate buffer (pH 8.3). After centrifuging the reaction mixture at 10,000 g for 10 min, enzymatic activity was determined in the supernatant by measuring absorbance at 382 nm. ACE-I inhibitory activity (%) was quantified in a concentration of 40 mg mL-1 and calculated as follow:
where, A represents absorbance in presence of ACEI sample, B absorbance of the control, and C absorbance of the reaction blank.
Anti-diabetic activity. In vitro α-amylase inhibition
Starch (2 mg) (substrate) (Dineshkumar et al. 2010) was suspended in a tube with 0.2 mL of 0.5 M Tris-HCl buffer (pH 6.9) containing 0.01 M CaCl2. The tube was boiled for 5 min and incubated at 37 °C for 5 min; 200 𝜇L PEE at 150 mg mL-1 were dissolved with 1 mL of 0.1% of dimethyl sulfoxide. Then 0.2 mL of each PEE was put in the tube containing the substrate solution, and 0.1 mL of porcine pancreatic amylase in Tris-HCl buffer (2 units mL-1) were added. The reaction was carried out at 37 °C for 10 min. The reaction was stopped adding 0.5 mL of 50% acetic acid in each tube. The reaction mixture was then centrifuged at 2 000 g for 5 min at 4 °C. The absorbance was measured at 595 nm. The results were expressed as percent inhibition of 𝛼-amylase through equation:
where, Ac+, Ac-, As, Ab are defined as the absorbance of 100% enzyme activity (only solvent with enzyme), 0% enzyme activity (only solvent without enzyme), a test sample (with enzyme) and a blank (a test sample without enzyme), respectively.
In vitro α-glucosidase inhibition
The α-glucosidase (50 μL, 2 U mL-1) (Dineshkumar et al. 2010) was premixed with 50 μL of PEE (at concentration of 0.5 mg mL-1) and incubated for 5 min at 37 0C. Then 1 mM para-nitrophenyl gluco-pyranoside (pNPG) (50 μL) in 50 mM of phosphate buffer (pH 6.8) was added to initiate the reaction. The mixture was further incubated at 37 0C for 20 min. The reaction was terminated by the addition of 125 μL of 1 mM sodium carbonate. The α-glucosidase activity was determined at 405 nm. The results were expressed as percent inhibition of α-glucosidase through equation:
where, Ac+, Ac-, As, Ab are defined as the absorbance of enzyme solution 100% enzyme activity (only solvent with enzyme), 0% enzyme solution activity (only solvent without enzyme), a test sample (with enzyme) and a blank (a test sample without enzyme), respectively.
Statistical analysis
The results were evaluated by analysis of variance, to determine significant differences between means (p < 0.05) which was determined by Tukey’s multiple range test with the statistical package Statgraphics® Centurion version 16.1.15. The correlation coefficient (r) between results (total phenolics, flavonoids, antioxidant and pharmacological activities) was determined from the Pearson correlation coefficient, which quantifies the linear association between two quantitative variables. This correlation was determined using Statistica 7.0 software.
RESULTS
Total phenols and flavonoids content
The percentage obtained of dry PEE from each one of the three raw propolis samples was 9.97, 5.56 and 6.66% from Tizimín, Huhí and Maxcanú, respectively. Table 1 shows the average of total content of phenols and flavonoids of the PEE of the three samples, collected in three different places in Yucatán Peninsula. A significant difference was found (p < 0.05) in both type of BC, in the three samples. Huhí sample had 85.64 mg CE g-1 dry extract of flavonoids, which was the highest among all three samples. Regarding total phenols content, also Huhí sample showed the highest amount with 25.95 mg GAE g-1 dry extract. The sample of Tizimín has the lowest content of phenols (1.27 ± 0.02 mg GAE g-1 dry extract) and Maxcanú the lowest of flavonoids (65.06 ± 3.48 mg CE g-1 dry extract).
Table 1 Means ± standard deviation of total phenols and flavonoids content of PEE of three localities from Yucatan, Mexico.
| Samples | Total phenols. (mg GAE/g-1 dry extract) |
Total flavonoids. (mg CE/g-1 dry extract) |
| Tizimín | 1.27 ± 0.02c | 71.22 ± 2.46b |
| Maxcanú | 11.06 ± 0.25b | 65.06 ± 3.48c |
| Huhí | 25.95 ± 0.99α | 85.64 ± 4.09α |
a-c Values with different letter in the same column indicate significant difference (p < 0.05)
Antioxidant activity
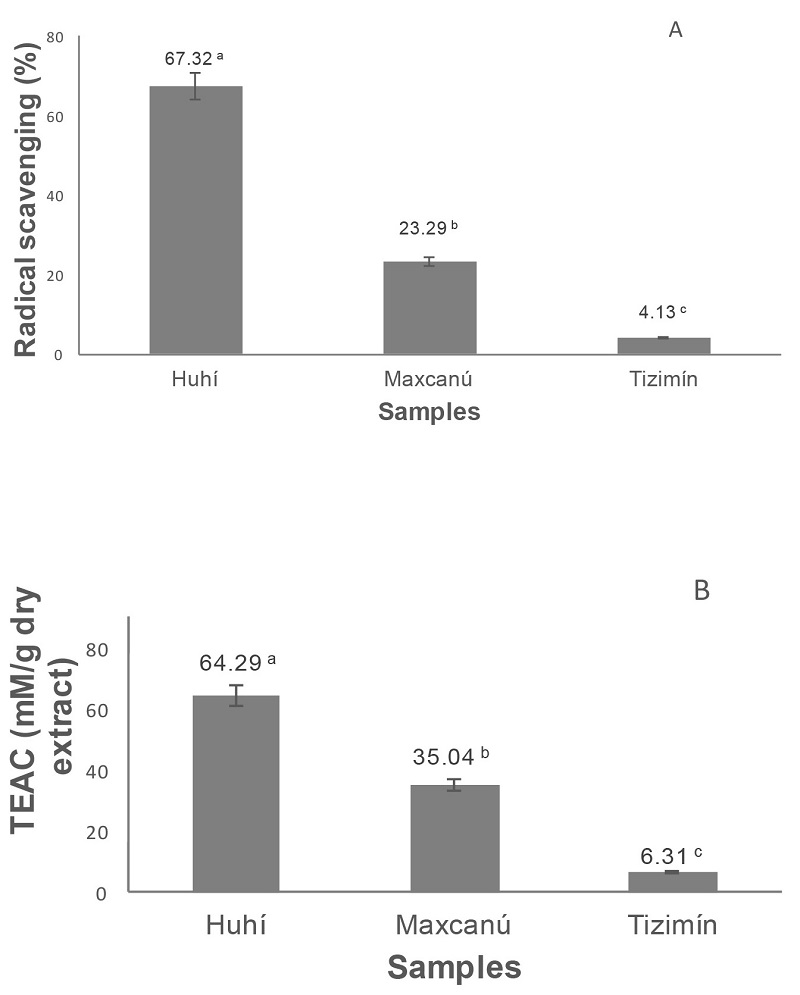
Figure 1 shows antioxidant activity, (ability scavenging free radicals) of propolis analyzed by two methods DPPH* and ABTS+ (TEAC). The three extracts possessed potent and diverse antioxidant activity with significant differences (p < 0.05). Propolis extract from Huhí exhibited the higher antioxidant potential in both methods (Fig. 1) 67.32 and 64.29%, respectively. Likewise, the PEE from Tizimín showed the lower DPPH* and TEAC antioxidant activity. Differences in antioxidant activity indicate that composition of propolis is significantly different.
Composition and chromatographic profile of PEE
Chromatographic profile of PEE of three samples is represented in Figure 2 and some identified compounds are in Table 2. The profile of PEE from Huhí has several compounds significantly higher concentration than the Maxcanú and Tizimín PEE. Compounds identified by HPLC reveal the occurrence of at least six flavonoids and ten phenolic acids (phenols). Five of them, epicatechin, sinapic acid, trans-cinnamic acid, kaempferol and isorhamnetim were identified in the three PEE samples, where epicatechin and p-coumaric acid were major constituent flavonoid and phenol observed in Huhí PEE. These results corroborate the difference in the chemical composition of PEEs.
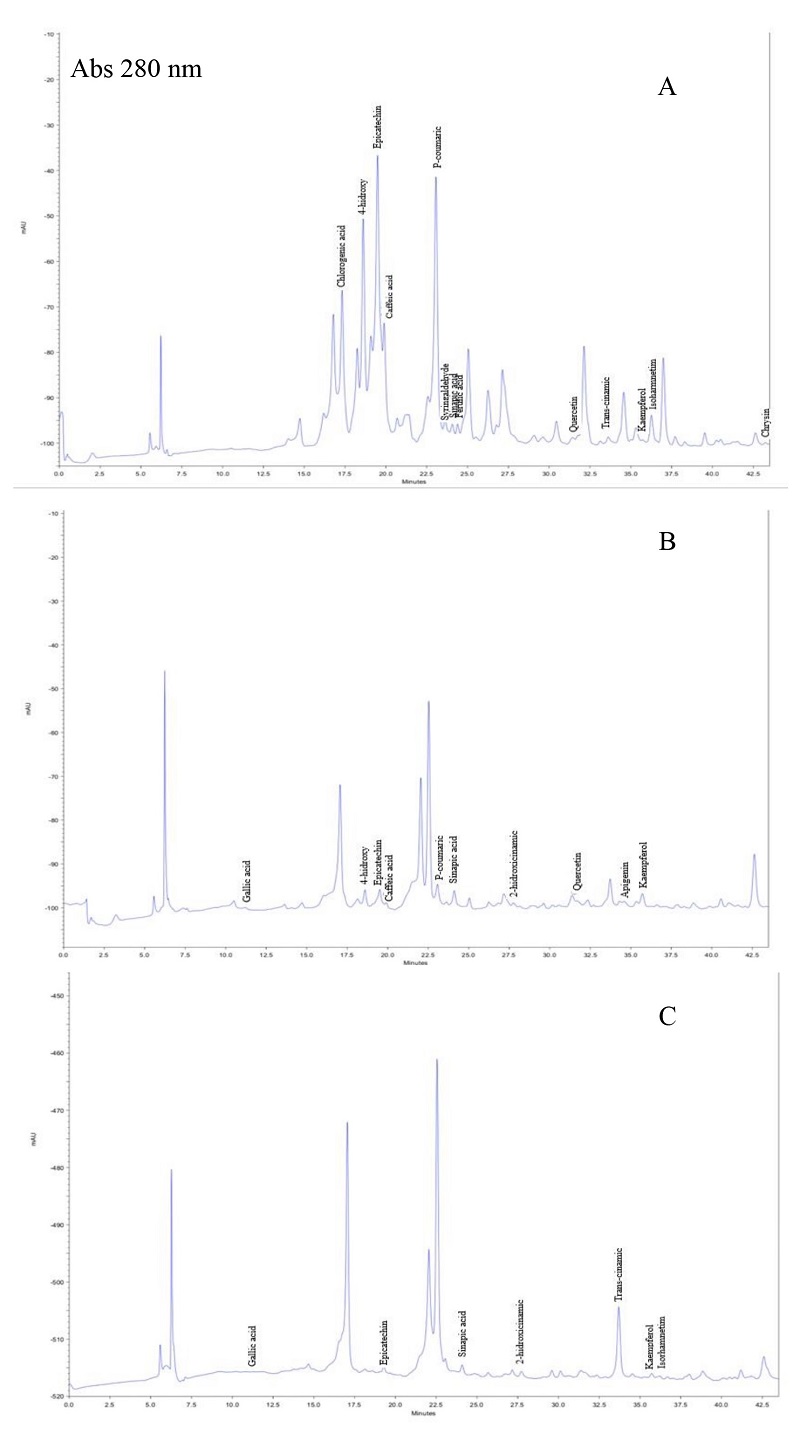
Figure 2 HPLC chromatograms at 280 nm of propolis ethanolic extract from: 2A, Huhí; 2B, Maxcanú; 2C, Tizimín respectively.
Table 2 BC identified using coincidence of retention times with known pure standards by HPLC.
| Peak No. | Retention | Retention time | Bioactive |
| time of PEE | of standards | compound | |
| 1 | 11.22 | 11.26 | Gallic acid |
| 2 | 17.31 | 17.27 | Chlorogenic acid |
| 3 | 18.61 | 18.47 | 4-hidroxy benzoic acid |
| 4 | 19.49 | 19.41 | Epicatechin |
| 5 | 19.89 | 19.8 | Caffeic acid |
| 6 | 23.06 | 23.06 | P-coumaric |
| 7 | 23.62 | 23.75 | Syringaldehyde |
| 8 | 24.06 | 24.08 | Sinapic acid |
| 9 | 24.39 | 24.5 | Ferulic acid |
| 10 | 27.75 | 27.56 | 2-hidroxicinamic |
| 11 | 31.78 | 31.62 | Quercetin |
| 12 | 33.61 | 33.5 | Trans-cinamic |
| 13 | 34.6 | 34.8 | Apigenin |
| 14 | 35.72 | 35.73 | Kaempferol |
| 14 | 36.26 | 36.34 | Isorhamnetim |
| 16 | 43.23 | 43.26 | Chrysin |
In vitro ACE-I inhibitory activity
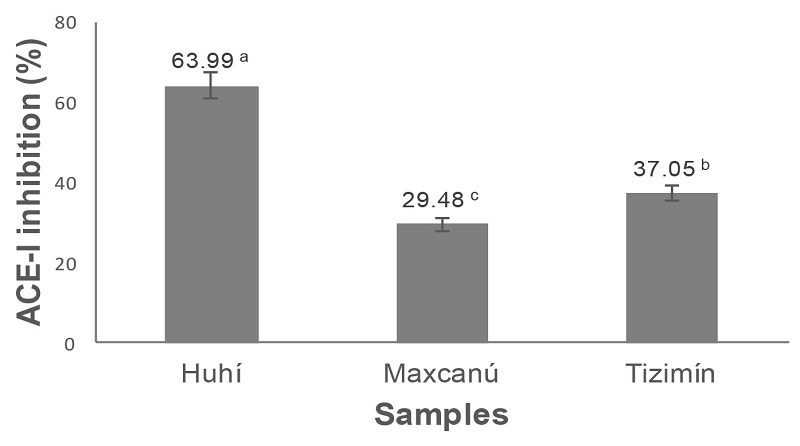
The Figure 3 shows a range of inhibition of ACE-I between 29.48 and 63.99%, while bioactive compounds present in Huhí PEE showed highest activity (63.99%) of the samples studied.
In vitro anti-diabetic activity
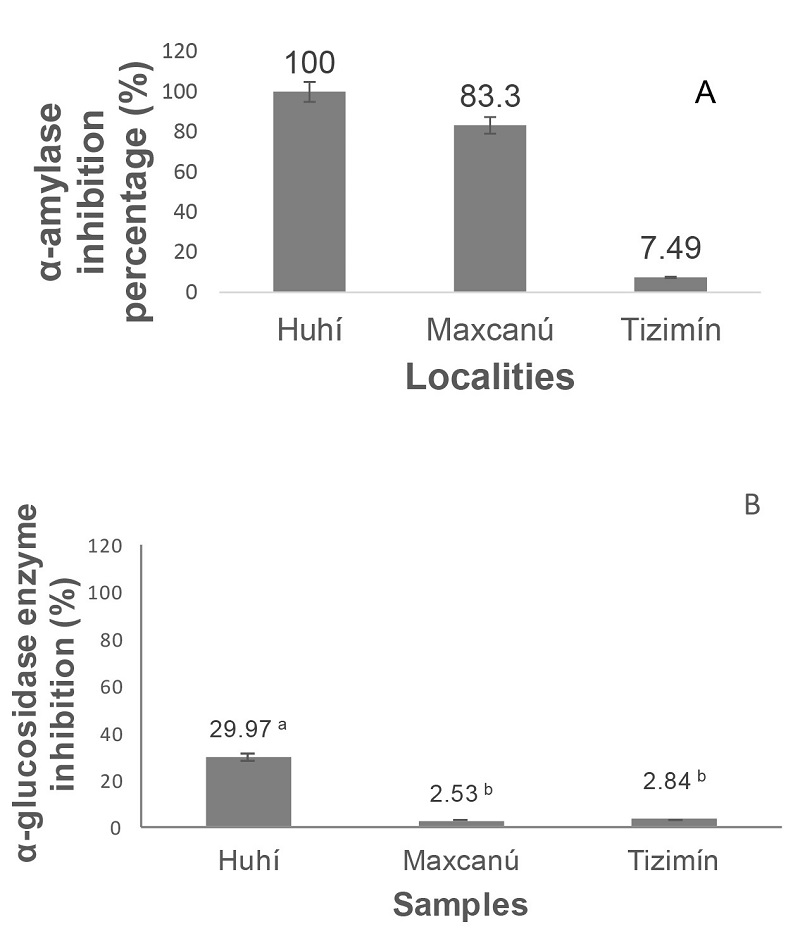
The bioactive compounds present in PEE, showed inhibition for a-amylase enzyme values of 7.49 ± 0.03%, 83.3 ± 0.04% and 100 ± 0.4% in Tizimín, Maxcanú and Huhí extracts, respectively (Figure 4), the significant difference in the inhibitory activity of the extracts is evident. Huhí PEE exhibited a higher inhibition percentage than that found in samples from India, Kerala (74.67 ± 2.57%) and Tamil Nadu (72.85 ± 3.89%) (Ramnath and Venkataramegowda 2017). Likewise, the Huhí PEE showed the higher inhibition of α-glucosidase activity value (29.97 ± 1.5%). Inhibitory activity of this enzyme of Maxcanú (2.53 ± 0.05%) and Tizimín (2.84 ± 0.06%) PEE were significantly lower.
Correlation
Antioxidant activity of PEE partly is due to its content of phenolic and flavonoids compounds, which is evident by the high significant values of linear correlation r (Perason’s correlation coefficient) found between phenolic and flavonoids concentration with antioxidant activity evaluated (Table 3), r = 0.99 and 0.75 with ABTS •+ and r = 0.99 and 0.82 for DPPH* respectively. The Inhibition of the enzyme activities (α-amylase, α-glucosidase and ACE-I) is significantly correlated with concentration of phenols and flavonoids. The significantly correlation suggests that antioxidant activity and inhibition of the enzymes are closely related to the phenol and flavonoid content of PEE.
Table 3 Pearson’s correlation values between different antioxidant activity parameters, total phenols and flavonoids, antidiabetic activity, and ACE-I inhibitory.
| Parameters | Total phenols | Total flavonoids | ABTS | DPPH | FRAP | α-amylase | α-glucosidase | ACE-I |
| Total phenols | 1 | |||||||
| Total flavonoids | 0.78 | 1 | ||||||
| ABTS | 0.97 | 0.75 | 1 | |||||
| DPPH | 0.97 | 0.87 | 0.98 | 1 | ||||
| FRAP | 0.81 | 0.97 | 0.78 | 0.90 | 1 | |||
| α-mylase | 0.88 | 0.49 | 0.93 | 0.84 | 0.51 | 1 | ||
| α-lucosidase | 0.88 | 0.96 | 0.86 | 0.95 | 0.99 | 0.63 | 1 | |
| ACE-I | 0.08 | 0.63 | 0.01 | 0.22 | 0.63 | -0.34 0.51 1 | 0.51 | 1 |
DISCUSSION
Propolis contains a variety of phenolic compounds, mainly flavonoids, phenolic acids, and phenolic acid esters. The difference in bioactive compounds such as total phenols (25.95 ± 0.99 mg g-1 dry extract) and total flavonoids (85.64 ± 4.09 mg/g dry extract) from Huhí PEE suggest that content of this bioactive compounds depends of flora found in the place where propolis was collected (Przybyłek and Karpinski 2019). Total phenols including p-coumaric acid, caffeic acid, ferulic acid have some biological activities such as antioxidant and anti-microbial. As the major constituents of propolis, flavonoids contribute greatly to the biological properties of propolis. The quantity of flavonoids is used as a criterion to evaluate the quality of propolis. Flavonoids have a broad spectrum of biological properties, such as anti-diabetic, antioxidant, antiviral and anti-inflammatory effects (Zhang et al. 2014). The concentration of phenols and flavonoids found in this work can be compared with results reported in propolis from several countries Argentine, Australia, Brazil, Bulgaria, Chile, China, New Zealand, Thailand, Uruguay and United States since the values of total phenols was between 31.2 - 299 mg g-1 and values of total flavonoids between 2.5 -176.0 mg g-1 of extract (Ahn et al. 2007).
The difference in bioactive compounds quantity present in the three PEE studied such as phenols and flavonoids are evident in HPLC profile chromatograms, the number and peak size. BC identified in this work are like found in some other works (Alencar et al. 2007, Salatino et al. 2011). Some of these compounds such as gallic acid, and caffeic acid phenethyl ester (CAPE), caffeic acid, chrysin, and quercetin, apigenin, kaempferol, have been associated with chemoprevention, antioxidant, and anti-hypertensive properties (Morais et al. 2010). The content of this type of bioactive compounds could be related to the antioxidant activity of propolis and to possible antihypertensive and adjuvant properties for the control of type 2 diabetes of propolis PEE from Yucatán.
The presence of free radicals can be deleterious to cells and may be related to the appearance of some diseases such as diabetes, arthritis, and others (Phaniendra et al. 2015). Antioxidant activity of propolis is associated with total phenols and total flavonoids content (Kasiotis et al. 2017). The antioxidant activity of PEE was evaluated with two different oxidant radicals, ABTS •+ (Trolox equivalent, TEAC mM g-1 dry extract) and DPPH* (% scavenging reaction of DPPH*). Huhí PEE sample has the highest values of antioxidant activity with both methods, ABTS (64.29 TEAC mM g-1 dry extract) and DPPH* (67.32% radical scavenging). The antioxidant activity is related to phenolic (25.95 ± 0.99 mg GAE g-1 dry extract) and flavonoids (85.64 ± 4.09 mg CE g-1 dry extract) content with which it is significantly correlated (Table 3), r = 0.99 and 0.75 with ABTS •+ and r = 0.99 and 0.82 for DPPH*, respectively.
Control of hypertension is one of the great challenges of society in countries such as Mexico. There are drugs that reduce blood pressure by inhibiting the ACE-I and show a positive effect in about 35-37% of patients. Some studies carried out to inhibit ACE-I, suggests that ACE-II also decreases which would have hypotensive effect (Li et al. 2013). Thus, the results obtained of PEE from Huhí and Tizimín (inhibition of 63.99 and 37.05% of ACE-I) provide solid evidence of the possible anti-hypertensive effects due to their active constituents. The In vitro ACE-I inhibitory activity was significantly correlated (r = 0.63) to total flavonoids content of PEE studied (Table 3). In one study has been found that some compounds, as pinocembrin and CAPE present in propolis extracts had vasodilator effect for low blood pressure (Granados-Pineda et al. 2018).
The inhibition of ACE-I by Huhí PEE, is promising application of propolis for the potential treatment of hypertension due the bioactive compounds present in this propolis, maily flavonoids (Teles et al. 2015).
The inhibition of α-amylase and α-glucosidase enzymes would delay the polysaccharides degradation which would decrease the absorption of glucose, as a result there is a reduction of post prandial blood sugar level. Hyperglycemia could be the cause of increasing the level of free radicals, leading to oxidative tissue damage and diabetic complications, which can be controlled by the natural antioxidants polyphenols compounds (Salah et al. 2017). In this study, the bioactive compounds in PEE evaluated from Huhí and Maxcanú were found to possess both favorable α-amylase (100 and 83.3%) and α-glucosidase (PEE from Huhí, 29.97%) inhibitory effects on starch breakdown in vitro. In general, the constituents of PEE studied produced a better α-amylase enzyme inhibition than α-glucosidase enzyme. Our results indicate that exist significant linear correlation between total phenols with α-amylase and α-glucosidase enzymes inhibition (r = 0.88 in both cases) and flavonoids with α-glucosidase enzymes inhibition (r = 0.96) which indicates that part of the inhibitory effect on action of these two enzymes could be explained by the content of phenolic and flavonoids compounds.
An effective strategy for the management of Type 2 diabetes is the inhibition of both enzymes to slow the absorption of carbohydrates, thus moderating the postprandial rise of blood sugar (Krentz and Bailey 2005). The possible control of type 2 diabetes through propolis extracts would have the advantage over currently used drugs as acarbose, since they would not have side effects that these have as bloating, flatulence, meteorism and diarrhea, as a result of an abnormal bacterial fermentation of undigested carbohydrates.
CONCLUSIONS
The results of this study about the composition and properties of propolis ethanolic extract confirm that its content of bioactive compounds can vary significantly depending on the region in which propolis is collected. It was found that the content of phenolic an flavonoids compounds are related to their antioxidant activity as well as their ability to inhibit (in vitro) the activity of the enzymes ACE-I, α-amylase and α-glucosidase, which is related to possible antihypertensive (vasodilator) capacities and for the reduction of the level of glucose in blood (antidiabetic), mainly the propolis collected at Huhí. This study shows the potential use of propolis as an adjuvant in the control of arterial hypertension and type 2 diabetes, although it is advisable to carry out more in vivo research in this regard.











 nueva página del texto (beta)
nueva página del texto (beta)