INTRODUCTION
Planktonic organisms are the basis of the food chain in marine and continental water bodies (Boltovskoy 1981). In marine aquaculture, the use of zooplankton as live food is essential and takes on greater importance during the larval phase, highlighting that it is the best nutritional alternative for the larvae, providing higher survival (Das et al. 2012). In the marine environment, copepods are the most dominant, abundant and widely distributed group of microcrustaceans on the planet, sometimes representing up to 80% of the total plankton (Melo et al. 2014).
In marine fish culture, rotifers of the genus Brachionus are the most used conventional live food for feeding larvae. As they are very active and small-sized organisms, they attract the attention of their predators, allowing them to be easily captured. In addition, by having a high reproduction rate, short life cycle and feeding based on algae, their cultivation is considerably facilitated (Prieto and Atencio 2008, Onyango 2019, Fuller 2020). However, these organisms do not necessarily complete the nutritional requirements for the optimal development of the marine fish larvae that consume them, generating limited growth. All this can lead to high mortality, possibly due to incomplete nutrition and poor development of the digestive system of the larvae (Luna-Figueroa and Arce 2017, Luna-Figueroa et al. 2018).
Mass production of copepods has been proposed as an alternative to good quality live food in aquaculture (Suárez-Morales 2000, Prieto et al. 2006, Suárez-Morales et al. 2009, Rasdi and Qin 2016, Hansen 2017, Martínez-Silva 2018, Hill et al. 2020). This proposal takes up the principle that the nutritional value of most copepods is high, and their movement patterns provoke a strong food response in larvae of diverse predatory fish species. Other advantages of using copepods as food are high digestibility, small size, and tolerance to good densities during culture. The simple fact that they are part of the natural food of larvae in the wild allows their use in marine fish culture to promote increases in growth, survival, and quality of the larvae that consume them (Prieto et al. 2006, Martínez-Silva 2018). Nonetheless, when working in aquacultural facilities, it is important to consider that both, rotifers enriched with fatty acids and copepods administered to the fish larvae, increase the possibility of larval survival. The copepods can be considered a nutritional supplement rich in fatty acids.
The quantity, temporality, size, and quality of live food during the larval period are critical to the successful production of marine fish fingerlings. An example of a culture strategy that combines several of these aspects is the so-called mesocosm culture, which has shown promising results by offering a variety of prey that facilitates the larval food transition by imitating what occurs in the wild, and where copepods are a fundamental element (Prieto et al. 2006, Rasdi and Qin 2016, Vu et al. 2017, Wang et al. 2017). The relationship between potential prey and larval selectivity can be approximated by the spatial-temporal coincidence of the two organisms at the spawning sites. An example of this is that the sea herring (Clupea harengus), the red snapper (Lutjanus campechanus), in its larval stage coexists with a high abundance of zooplankton (Álvarez-Fernández et al. 2015, Grüss et al. 2018, Heyman et al. 2019). The Gulf of Mexico (GM) is no exception because it is also considered an important marine fish spawning area determined by its abundance of larvae (Flores-Coto et al. 2009, Heyman et al. 2019). Zavala-García et al. (2016) recorded the largest zooplak-ton biomass ocurring in GM locations during the same season when spawning grounds of Centropomus spp where reported by Hernández-Vidal et al. (2014). This event suggests that some of the zooplankton species present, could play an essential role in nutrition and consequently in larval recruitment in the area.
The copepod Apocyclops panamensis is a species present in these spawning areas on the coasts of the Gulf of Mexico, so that may conform part of the diet of fish larvae in the region. Preliminary studies indicate that it has the potential to be used as live food since it is resistant to manipulation in captivity, been proposed as a candidate for mass cultivation (Phelps et al. 2005, Lindley et al. 2011, Santhosh et al. 2015, Hill et al. 2020). However, the environmental conditions for developing the biotechnology for cultivation on a pilot scale are still unknown. For this reason, the objective of this work was to define the effects of salinity and temperature on the population growth of A. panamensis under an aquaculture management scheme that could optimize and eventually allow to scale-up production and prove its effectiveness as live food for marine fish species present in the same distribution range.
MATERIALS AND METHODS
This research was carried out in the facilities of the Tropical Aquaculture Laboratory (TAL) of the Biological Sciences Academic Division at the Universidad Juárez Autónoma de Tabasco during August and September of 2019.
Collection and preliminary cultivation of zooplankton
Three zooplankton collection trips were made to the coastal zone in Jalapita, municipality of Centla, Tabasco, Mexico, identified as centropomid spawning areas (Gilmore et al. 1983, Hernández-Vidal et al. 2014). The collection sites covered an area ranging from 18○ 28’ 34.71” N - 92○ 58’ 7.34” W to 18○ 26’ 26.43" N - 93○ 8’ 4.58" W, the furthest collection site is 4.7 km from the coastline, and the nearest is approximately 0.2 km from the mouth of the Mecoacán coastal lagoon. Surface trawls (1-2 m deep) were conducted using a 120 µm mesh plankton net. The zooplankton was placed in 500 mL -1 capacity glass jars with water from the sampling site and then moved to the TAL at low temperature in an icebox. In the laboratory, the living material was screened with a 500 µm sieve to remove larger organisms and predators. The remaining organisms were placed in two fiberglass culture tanks with a capacity of 70 L and were maintained at an average temperature of 25.7 ± 1.13 C and pH of 7.96 ± 0.05 using seawater at the same salinity of the collection site (32‰). The water was previously filtered with 2µm and treated for 10 min with a commercial solution of sodium hypochlorite to reach the concentration of 45ppt and later neutralized by adding 250 mg of sodium thiosulfate L -1. The cultures were fed daily with a mixture of the microalgae Nannochloropsis oculata, Tetraselmis chuii, and T. suecica supplying a total of 8 million cells per experimental unit, equivalent to a density of 20 000 cells mL -1. After 20 days of culture, the prevailing zooplankton was recovered with a 50 µm sieve that was again maintained for 30 days in conditions similar to those described above.
Isolation and identification
The isolation of the prevailing copepod species began by separating adult specimens. These organisms were concentrated using a 50 µm sieve and under the stereoscopic microscope, and ten adult organisms were isolated. The isolated organisms were kept in 1L-flasks and fed with the previously mentioned algal mixture. This separation of adult organisms was carried out on three occasions, and the organisms were reseeded in new flasks. Samples were fixed in 96% alcohol added with two drops of glycerin in 5mL-tubes. Subsequently, they were sent for determination to the Department of Systematics and Aquatic Ecology at El Colegio de la Frontera Sur (ECOSUR) Chetumal Unit, who determined that the isolated species was Apocyclops panamensis. The primary culture was obtained by progressively increasing the volume from the 1L-culture flasks and maintaining the culture conditions previously described.
Experimental design
To evaluate population growth, a completely randomized factorial experiment was carried out with three temperature levels (24, 28, and 32○ C) and three salinity levels (28, 32, and 36‰), resulting in nine treatments, each with six replicates. The experimental units consisted of 0.5L capacity glass flasks containing a working volume of 400 mL. The culture containers were disinfected by washing with a 0.01% sodium hypochlorite solution. To achieve and maintain desired temperatures, the experimental units were placed inside 100L polyethylene tanks, equipped with two 500-watt and controlled with a thermostat (AQUA-KRIL® 4160). Seawater (35‰) was used, previously treated with sodium hypochlorite, and ter was diluted with sterilized freshwater to reach the salinity of 28 and 32%, to adjust to 36‰, commercial sea salt was used (Coral Pro-Red Sea ®). All experimental units were maintained under a photoperiod of 12h:12h (light-dark) controlled with an automatic timer (TEMP 24-HE, STEREN ® ). pH and water temperatures were measured daily using a probe (EcoSense pH10A, YSI USA), while a refractometer was used to measure salinity (AquafaunaTM Bio-Marine Inc.). Ammonium (NH3), nitrites (NO2), and nitrates (NO3) concentration were measured at the beginning and, subsequently, every five days by colorimetric kits using an aquaculture photometer (HI 83203, HANNA Instruments).
Copepod stocking
A cohort of nauplii was obtained from the main population and separated using a 200 µm mesh sieve to discard copepodites and adult organisms. The nauplii were placed in a glass beaker with sterilized seawater and the density reached was estimated. Before stocking, the seeds were first acclimated to the desired salinity by decreasing or increasing one unit per hour, and then to temperature, by placing the nauplii in 1L flask one hour prior to stocking in each tank with the corresponding temperature. Each experimental unit was stocked with one ind mL -1 for a total of 400 individuals per bottle based on the recommendations of Velásquez et al. (2001).
Feeding
The feeding was carried out using the microalgae Tetraselmis chuii, according to Velásquez et al. (2001). Initially, each unit was supplied with a total of 8 million cells, equivalent to a density of 20 000 cells mL -1. Cultures were used in their maximum growth phase (5-6 days), and the algal density was maintained by counting twice a day and adjusting the preset density when necessary. Microalgae cultures were produced in the Live Food Production Area of the Tropical Aquaculture Laboratory (LAT) at DACBiol using commercial culture medium (PROLINE®), the cultures were maintained at constant illumination with fluorescent lamps from 2 000 to 2 500 lux and at a temperature of 24 ± 1.0 ○C.
Counting
Counts of copepods were performed every third day, taking seven one mL samples from each experimental unit. The sample was counted using a Bogorov counting chamber (Wildlife Supply model 1810-B20El) under a stereoscopic microscope (ZEISS ® model Stemi DV4). The stages identified in the culture count were nauplius, copepodite, non-ovigerous female, ovigerous female, and adult male. In addition, the eggs present in the ovarian sacs of the females were counted. The last evaluation of the population was carried out on the 14th day after sowing, which concluded the experiment.
Statistical analysis of data
The number of organisms per stage was calculated for each treatment, and with the sum of all stages, the value of total copepods (TC) per liter was estimated. After normality and homogeneity of variances were verified (Bartlett’s test), the counts obtained at the end of the experiment were compared using a factorial analysis of variance (ANOVA). Significance was determined at the 95% confidence level (α = 0.05). All ANOVAs were performed using the software Statgraphics Centurion XVIII ® . The graphical representation of the data was done in SigmaPlot v.11 ® package. Results are presented in average values per Liter ± Standard Deviation.
RESULTS
Environmental conditions during the experiment
The environmental conditions recorded throughout the experiment were favorable for the development of copepods (Table 1). The temperature varied slightly around the experimental values. The average temperatures prevailing along the experimental units were 24.24 ± 0.19, 27.86 ± 0.43, and 31.75 ± 0.27. We managed to keep salinity close to the values designated for the study (28 ± 0.18, 32 ± 0.34 and 36 ± 0.32 by monitoring twice a day and replacing evaporated water.
Table 1 Seawater parameters (Mean ± DE) observed under different temperatures (T) and Salinity (Sal). Nitrites (NO2), nitrates (NO3), and total ammonia nitrogen (TAN) were measured every third day, pH, and dissolved oxygen (DO) were measured daily.
| T(○C) | Sal (ppm) | NO2 (mg L -1) | NO3 (mg L -1) | TAN (mg L -1) | pH (IU) | DO (mg L -1) |
| 28 | 2.30 ± 1.54 | 2.47 ± 4.27 | 0.37 ± 0.34 | 8.12 ± 0.08 | 7.17 ± 0.33 | |
| 24 | 32 | 0.67 ± 0.58 | 4.87 ± 4.24 | 0.49 ± 0.58 | 8.11 ± 0.06 | 6.60 ± 0.08 |
| 36 | 0.33 ± 0.58 | 0.63 ± 1.10 | 0.47 ± 0.55 | 8.10 ± 0.05 | 6.54 ± 0.12 | |
| 28 | 2.47 ± 2.54 | 1.33 ± 2.31 | 0.36 ± 0.35 | 8.17 ± 0.09 | 6.99 ± 0.24 | |
| 28 | 32 | 1.97 ± 0.95 | 1.77 ± 1.91 | 0.51 ± 0.50 | 8.15 ± 0.05 | 6.92 ± 0.30 |
| 36 | 3.33 ± 2.08 | 2.17 ± 2.20 | 0.81 ± 0.95 | 8.18 ± 0.08 | 6.36 ± 0.16 | |
| 28 | 1.67 ± 2.08 | 1.47 ± 2.04 | 0.34 ± 0.30 | 8.23 ± 0.22 | 5.80 ± 0.42 | |
| 32 | 32 | 1.33 ± 1.50 | 1.27 ± 2.19 | 0.65 ± 0.61 | 8.19 ± 0.09 | 6.30 ± 0.18 |
| 36 | 2.33 ± 3.21 | 3.57 ± 3.10 | 0.56 ± 0.55 | 8.09 ± 0.22 | 6.10 ± 0.11 |
Total counts
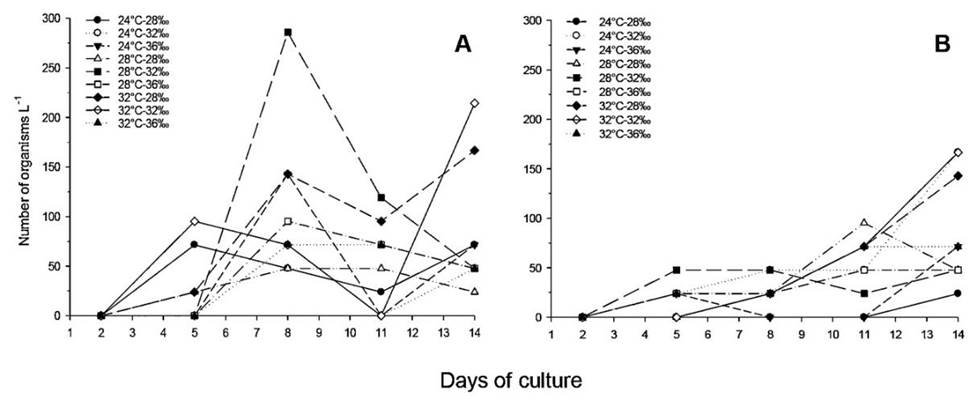
Results from the factorial ANOVA indicate that the temperature had a significant effect on the overall abundance of organisms in the population assessed at day 14 (p = 0.02) and suggests that the evaluated salinities did not have a significant effect on A. panamensis production (p = 0.06). In this analysis no effect of the interaction of the factors studied was observed (p> 0.05). The effect of temperature was that, at a temperature of 32○ C, more organisms were obtained (888.89 ± 414.28 ind L -1) being significantly lower at temperatures of 24 and 28○ C (293.65 ± 285.72 and 357.14 ± 264.70 ind L -1, respectively) (Figure 1A). Although salinity was not statistically significant, a distinctive pattern of decrease in the total number of copepods (TC) can be observed as salinity increases, resulting in 722.22 (± 428.57), 500.00 (± 357.14) and 317.46 (± 214.29) ind L -1 for salinities of 28, 32 and 36‰, respectively (Figure 1B). At the level of specific treatments, the results of TC indicate that the best productivity of this species at the end of the experiment is reached in treatments 32○ C-28‰, with a production of 1 502.00 ± 457.74 ind L -1 and 32○ C-32% where 904.76 ± 576.17 ind L -1 were obtained (Figure 2). When evaluating the effects on the number of copepodites, nonovigerous females, ovigerous females and males at the end of the experiment, no statistically significant effects of the factors evaluated or their interaction were observed (p > 0.10 in all cases). The specific observations for each group of organisms allowed to follow the trends that contribute so that the treatments 32○ C-28‰ and 32○ C-32‰ have reached the highest total of the experiment.
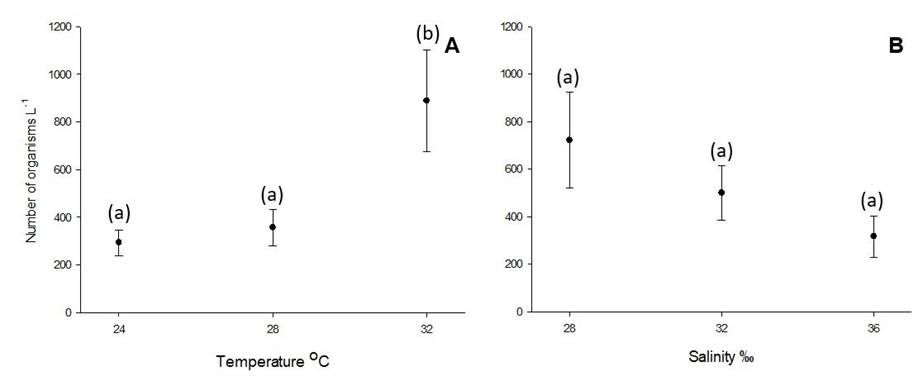
Figure 1 Mean values (±Standard error) of total copepods observed in the three temperatures evaluated (A) and the three salinities (B). Different letters indicate statistically significant differences (p < 0.05) between temperatures. The sample size per group is n = 18.
Nauplii
The factorial ANOVA for the number of nauplii indicated significant effects of temperature (P = 0.006) and salinity (p = 0.001), with no interaction between these factors (p > 0.10). The 32○ C-28‰ treatment had the highest average number of nauplii recorded at the end of the experiment, reaching an average of 738.09 ± 602.15 ind L -1. The rebound of this treatment began on day eight, when the population reached 452.38 ind L -1, by day eleven, the population already had 1 142.86 ind L -1. This trend was also observed in the 32○ C-32‰ treatment, although it was not as pronounced as the previous treatment. In the rest of the treatments, the highest populations did not exceed 400 nauplii L -1, the lowest results being those associated with temperatures of 24○ C (Figure 3A).
Copepodites
In general, all treatments showed ups and downs along the trial. The only tendency of increase was observed is in treatment 32○ C-28‰ for day 11, having an average of 190.47 ± 233.28 ind L -1 and reaching 333.33 ± 147.54 ind L -1 on day 14. All other treatments remained below 150 ind L -1 (Figure 3B).
Adult females
The trend analysis of females includes nonovigerous females and ovigerous females, showing high variability in the recorded data. The highest values at the end of the experiment correspond once again to treatments 28 ○C-32 ○C-32h and 32 ○C-28h averaging 166.67 ± 167.01 and 119.04 ± 140.46 ind L1, respectively. The rest of the treatments presented less than 75 ind L -1 (Figure 4A).
Adult males
The number of adult males remained constant throughout the experiment, with consistent increases observed towards days 11 and 14 in three treatments (24○ C-28‰, 32○ C-28‰, and 32○ C-32‰) however, none of them exceeded 170 ind L -1. The 24○ C-28% treatment presented the lowest average number of males with 23.81 (± 3.04) ind L -1 on day 14 of the experiment (Figure 4B).
Female:male ratio
This ratio fluctuated considerably throughout the experiment. During the first five days, no adults were observed in the samplings; these were differentiated from day eight. The highest proportion of females was observed in treatments 28○ C-32‰ and 32○ C-28‰ reaching up to six females per male. These values decreased on day 11, with the highest proportion in the treatment being 28○ C-32‰ with five females per male. At the end of the experiment (day 14), the ratio female: male varied in treatments between 0.29 and 3.0, averaging between treatments 1.1 females per male.
DISCUSSION
The population trends observed during 14 days starting from a cohort of nauplii indicate that Apocyclops panamensis grow well under temperatures near 32○ C and salinities ranging from 28 and 32‰ A. panamensis is one of the few species of cyclopoids cultured in the laboratory and recommended for aquacultural practices; however, despite this recommendation, little is known about the specific environmental requirements for this copepod species abundant in the neotropical region (Suárez-Morales et al. 2004). The fact that the two best treatments correspond to the temperature of 32○ C indicates that this species has better performance under the upper range of temperatures measured in warm-tropical environments. In recent years, we have recorded an average of 29.5○ C for the warmest months of the year (September-October) near the sites where A. panamensis was collected for the current study, and this corresponds a shallow coastal habitat where is possible to reach high temperature during sunny and wind calm days. Our study suggests that A. panamensis like most copepods display a wide tolerance range for temperature fluctuations.
There is a variety of information regarding the effects of temperature on copepod populations, but the leading theory focuses on the direct effects of temperature on fecundity and growth. Warm temperatures promote a fast life cycle, which can translate into high population growth in a short period; this is possible because the duration of the developmental stages and molting rates are physiological processes that are primarily dictated by temperature (Hirst and Bunker 2003, Chailinn et al. 2018). Proof of this is that many tropical Cyclopoid copepods have life cycles lasting from four to five days, making them susceptible to mass cultivation (Su et al. 2005, Rasdi et al. 2018). While some species can acclimatize to temperatures outside their normal ranges without inhibiting their reproductive potential (Verbitsky et al. 2017), other species make reproductive adjustments, for example, Acartia tonsa - a temperate climate species - decreases the number of eggs produced by females in cold temperatures (Hansen et al. 2010). Similar results have been reported for A. distans, a species belonging to the same genus, whose highest population growth was achieved under a temperature range of 26 to 33.8○ C.
Salinity is another of the environmental parameters that strongly influence the ecological and biological responses of copepods (Pan et al. 2016). In this experiment, it becomes evident that A. panamensis is well adapted to a relatively wide range of salinities; however, population growth displays best results at 28‰, particularly for the naupliar stage. This species is considered euryhaline since it has been reported in environments ranging from the oligohaline condition that occurs in coastal lagoons and estuaries to hypersaline waters in semi-desert areas of North America (Pérez et al. 2006, Reid and Hribar 2006, Annabi-Trabelsi et al. 2019). The wide tolerance of the species has also been demonstrated by being collected in salinity of 6.6h and later successfully acclimatized to 30h (Lindley et al. 2011, Roy and Venkataraman 2018). It is inferred that it is a species that tolerates wide ranges of salinity, with a preference for brackish water as observed in this experiment. Even though euryhaline species can survive under a wide range of salinities, reproductive performance can be affected severely depending on this parameter leading to unsustainable population growth and ultimately result in copepod mortality (Pan et al. 2016). Reid et al. (2002) and Fuentes-Reinés and Suárez-Morales (2015) considered that the genus Apocyclops is so ubiquitous that can be observed dominating plankton communities in coastal lagoons, saline lakes, and coastal marshes. However, it is frequently associated with salinities under 30h. In our experiment, all treatments having 32‰ resulted in reduced production of nauplii. This response to higher salinity levels is explained by Kimmel and Bradley (2001) and Pan et al. (2016) as a result of a shift in the energy budget for reproduction towards energy needed for adjusting the metabolism required for osmoregulation. The fact that we have registered population growth in the various conditions evaluated is proof of the vast adaptive capacity of A. panamensis.
In general for this group of organisms, it is known that adults have tolerance to several factors considered adverse as well as their latency stages since this facilitates dissemination by means of birds and wind, which could be the explanation of the successful colonization of contrasting environments in which they have been found, although little is known about the physiological and ecological mechanisms that allow copepods to reside in these environments (Anufriieva 2015).
In the best treatment of this study (32○ C-28‰), it was observed that the population grew 3.4 times in 14 days, reflecting rapid growth. These results are similar to other studies where Cyclopoid production has commonly been generated from 1 to 5 times the amount inoculated (Pérez et al. 2006, Farhadian et al. 2008, Ruiz-Guzmán et al. 2012). In an extreme case, Velásquez et al. (2001) reported a growth in the culture of A. distans of up to 27 times what was stocked for ten days using ambient temperature (26.0 - 38.8○ C), salinity around 40h and total darkness for their best treatment. The high productivity observed in our treatments mainly reflects the number of nauplii obtained, which are the objective for the aquaculture of fish larvae.
The obtained productivity of 738 nauplii L -1 is a moderate nauplii production when compared to values obtained by Phelps et al. (2005) in the experimental culture of A. panamensis using adult copepods in initial densities of 320, 1 280 and up to 5 120 adults L -1 for a period of 4 to 9 days. In that study, the author produced 16 942 nauplii L 1 in the maximum density of adults stocked. These differences could be atribuible to culture procedures and stages stocked, but suggest potential for nauplii production in A. panamensis under our defined environmental conditions.
Another factor that could have contributed to the productivity observed in A. panamensis is the type of food used. Tetraselmis chuii is considered an excellent food alternative for copepods, because they are mobile flagellated algae of considerable size, having a notorious preference in comparison with nonmobile algae. Velásquez et al. (2001) obtained the best production values of nauplii in A. distans using Tetraselmis chuii when compared with N. oculata. The cell density used in this study can be considered as low (20 000 cell mL -1) compared to other studies where 50,000 cell mL -1 (Pérez et al. 2006) to 600 000 cell mL -1 (Mujica et al. 1995) are used. Velásquez et al. (2001) used algae densities of 300 000 cell mL -1 of T. chuii to feed A. distans, while Ruiz-Guzmán et al. (2012) obtained the best results in the cultivation of Cyclopina sp. with the combination of T. suecica and Isochrysis galbana. The cell density of 600 000 cell mL -1, gave favorable results in the culture of Tigriopus sp. supplying Nannochloropsis sp. (Mujica et al. 1995). Phelps et al. (2005) cultivated A. panamensis using a cell density of 500 000 cell mL -1 using the microalgae Isochrysis galbana. The production of A. panamensis could be improved by using a varied diet, possibly favoring the productivity of copepod crops compared to feeding using a single species. This can be attributed to the high content of polyunsaturated fatty acids provided by the mixtures (Farhadian 2008). Alternatively, these algal diets can be improved by supplying other food sources such as flours of vegetable and animal origin, as well as inorganic fertilizers and using culture protocols such as mesocosm resulting in a good nutritional profile (Phelps et al. 2005, Prieto et al. 2006).
Copepods play an important role in the initial stages of development of marine fish because they constitute their main natural prey, in addition to having a high nutritive content, easy digestion and adequate transfer of nutrients, making them an important food source in aquaculture (Ruiz-Guzmán et al. 2012). Copepod nauplii are a rich source of free amino acids (FAA) and contain more than twice the amount of FAA than Artemia, and higher levels of HUFA (Naess et al. 1995, Rayner et al. 2017). Proof of this nutritional value is that copepods are used in the larval stage of turbot Scophthalmus maximus (Bruno et al. 2018) Atlantic cod Gadus morhua (Karlsen et al. 2015), red snapper Lutjanus campechanus (Phelps et al. 2005) and the gilt-head sea bream Sparus aurata (Mona et al. 2019). All these species proved to be success-ful predators of all stages of copepods of the orders Harpacticoida and Cyclopoida, demonstrating the potential of copepod cultivation by improving the nutritional quality of the diet and decreasing dependence on rotifers and artemia (Vanacor-Barroso et al. 2017). Data for FAA content in A. panamensis indicate that nutritional profile is similar to other copepod species used actually on larviculture (Lindley et al. 2011).
Copepods can be cultivated at high densities, and that their cultivation is relatively easy (Ribeiro and Souza-Santos 2011). They have even been successfully cultivated in recirculation systems where the production has been exclusively of nauplii and copepodites, stages that are used in the feeding of marine fish larvae (Buttino et al. 2012). In a recent study, we found that A. panamensis maintained at 28‰ and 32○ C accomplishes its life cycle in six days (data not shown) and reached 11 600 copepods L -1 in a 1 000 L tank. With these preliminary results, A. panamensis can be considered as a good candidate for mass culture protocol evaluations in order to determine conditions for pilotscale production (Ribeiro and Souza-Santos 2011, Hansen et al. 2017). Previous experiences by Phelps (2005) in A. panamensis cultured in earthen ponds indicate feasibility for mass production on this species; however, more information about the optimal conditions of variables in the culture system is necessary. The results presented here provide valuable information for A. panamensis culture, because the definition of preferred temperatures and salinities for a particular species, provide conditions where the organisms are subject to minimum stress; their physiological functions are optimized, providing a maximum population growth (Nichelmann 1983, Verbitsky et al. 2017). These data can be suitable for production protocols evaluations.
CONCLUSIONS
The species Apocyclops panamensis has a preference for warm waters (32○ C) and medium salinity (28-32‰). The high production capacity of A. panamensis observed in this experiment allows visualizing the potential for scaling up production and evaluation as live food for larval culture of tropical fish. Nauplii and copepodites can be an alternative for feeding larval stages of marine fishes due to their small sizes. Another convenient feature is that it has a short life cycle that makes them susceptible to rapid mass production. We consider that A. panamensis is a species suitable to be used as live food; however, it is required to demonstrate its viability for mass production at pilot scale and preference by larvae of different species of fish.











 nueva página del texto (beta)
nueva página del texto (beta)