Introduction
Reproduction in ruminants is influenced by several factors such as species, breed, physiological State, nutrition, and others. Within these factors, nutrition is both a requirement and a management tool, while dietary fat is an important source of fatty acids and energy accordingly. However, for strategies of lipid supplementation, it is important to consider the percentage of inclusion in the diet. In ruminants, oils rich in linoleic acids at 4% of the diet on a dry matter basis, do not show deleterious effects on productive performance (Roy et al. 2017). The mechanisms by which oil improves animal reproductive capacity were established as energetic and efficiency, by avoiding de novo synthesis of several compounds. However, an improvement in energy status may be mediated by changes in metabolic hormones (Scaramuzzi et al. 2006). Lipid levels consumed in the diet are associated with a modification in lipid metabolites in plasma, the increase in ovarian steroid secretion and other enzymes involved indirectly in reproductive processes of ruminants (Gulliver et al. 2012). Salas et al. (2011) mention that higher dietary lipids are directly related to an increased concentration of serum cholesterol and high-density lipoproteins in follicular fluid. Cholesterol is a precursor to estradiol synthesis and progesterone and therefore may alter their blood serum concentration, affecting positively or negatively reproductive aspects (Rahbar et al. 2014). Insulin could be considered another important metabolic hormone in the reproduction of the ewe; insulin is key on signaling the metabolic status of the animal, and in this context, can alter the frequency and concentration of LH and thus the ovarian response when interacting with glucose and leptin (Vinoles et al. 2005, Velazquez et al. 2008). In ruminants, insulin exerts a direct action on the membrane of granulosa cells, an action that may be required for the development of an optimal steroidogenic status (Dupont et al. 2014), that brings a better development of Corpus luteum cells, a reduction in the synthesis of PGF2α (Mattos et al. 2000) and hence a delay in luteolysis (Williams 1989). The objective of the present investigation was to evaluate the inclusion of corn oil in the diet of sheep and its effect on cholesterol, insulin, estradiol, and progesterone blood serum profiles.
Materials and methods
This experiment was performed according to the procedures established by bioethics committee of the Universidad del Papaloapan (CBUNPA/2016-03). The study was carried out during the spring season at the experimental area campus Loma Bonita, Oaxaca, located in the geographical coordinates 95° 53' North Latitude and 18° 06' west longitude, at a height of 30 meters above sea level. Twenty-one adult multiparous, non-lactating Pelibuey ewes were used, with a body condition (BC) at the start of the experiment of 2.7 ± 0.2 points on a scale of 1-5 (Russel et al. 1969) and body weight of 33.9 ± 1.57 kg. The sheep were confined for a period of 28 days, and randomly distributed into three treatments, isoenergetic (8.9 MJ d-1) and isoproteic (10.5% CP), differing on corn oil level used: T0: (0%, n = 7), T3: (3%, n = 7), and T6: (6%, n = 7). Diets were balanced according to requirements for sheep (ARFC 1993).
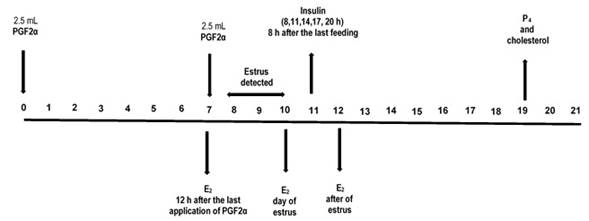
The estrus synchronization protocol (PGF2α: Dinoprost, Lutalyse, Pfizer) and blood sampling times are shown in figure 1. The blood samples were collected (10 ml) from each ewe by jugular vein puncture using EDTA vacutainer tubes (10%). The samples were kept under refrigeration for less than 60 min, centrifuged at 1 460 g for 10 min, and plasma was extracted and stored at -20 °C until analysis.
Hormone measurement: Insulin was determined by radioimmunoassay (Insulin Cout a Count, DPC, USA). Coefficients of variation for intra and inter assay insulin were 11.1 and 13.5%, respectively. Progesterone (P4) was analyzed by radioimmunoassay using a commercial kit (DPC, TKPGX, LAG, USA.), the intra and inter assay coefficient of variation was 6.54 and 2.92%, respectively, cholesterol: an enzymatic method was used (Sera Pak Plus, Bayer, Germany) and estradiol (E2): was analyzed by radioimmunoassay (DPC, California, USA).
Plasma cholesterol and progesterone were analyzed using a one-way ANOVA; insulin and estradiol were analyzed using a repeated measures two-way ANOVA (mixed model), where treatment and the time interval were the fixed effects. Data analysis were performed using the statistical software GraphPad Prism 5.
Results and discussion
The sheep weight at the beginning and end of the experiment, average daily weight gain (DWG) and BC did not vary on any the level of corn oil in the diet, and the daily weight gain did not show differences between treatments either (p > 0.05). Since diets were isoenergetic and isoproteic, and calculated for a positive nutrient balance, the ewes expressed positive DWG. However, as the oil in the diet increased, DWG did the opposite (T0: 72.57 ± 0.02, T3: 63.00 ± 0.01 and T6: 48.85 ± 0.02). It has been reported that the addition of fat sources in excess of 6% of the diet on a dry matter basis, causes a depression in dry matter fiber and energy digestibility (Hess et al. 2008), and changes the pattern of fermentation (Fiorentini et al. 2015). Jenkins et al. (2002) fed diets based on corn and soybean oil to grazing sheep, and observed a decrease in the ruminal acetic: propionic ratio. In this sense, feeding fat to the ruminant animal induces a lower energy efficiency and a greater proportion of acetic acid. Otherwise, an increase in the molar ratio of propionic acid generally results in a better DWG (Al-Arif et al. 2016).
The serum concentration of progesterone and cholesterol was similar between treatments T3 and T6, however, there was a difference (p < 0.05) compared with the control group (T0) in the luteal phase of the estrous cycle (Table 1). At higher oil levels in the diet, the level of cholesterol and progesterone concentrations rised. These responses corn oil supplementation, increases the energy concentration of rations and thus promotes the production of steroid hormones (Santos et al. 2008). There is evidence that indicates that after supplementation with lipids, blood serum and follicular cholesterol (Staples et al. 1998) and progesterone increase (Moriel et al. 2014), resulting in a longer middle life of the Corpus luteum (Fontona and Torre 2016). Ghoreishi et al. (2007) reported that an increase of plasma progesterone in sheep supplemented with fatty acids is especially important when these concentrations are maintenance in the luteal phase of the estrous cycle, because it generates a positive effect on pregnancy establishment and maintaining, since cholesterol is necessary for the synthesis of progesterone. For that reason, the increased availability of cholesterol generates a positive effect on reproductive behavior. Wehrman et al. (1991) reported that luteal cells from cows fed with 6.6% lipids in the diet have an increased secretion of pregnenolone and progesterone compared to those who received 2.2%. Another factor to consider is the effect exerted by the fatty acids in the synthesis of prostaglandins, which may serve as precursors or inhibitors of the synthesis of PGF2α depending on the fatty acid concentration in tissues where they are synthesized (Patterson et al. 2012). In sheep, uterine epithelial cells have shown a reduction up to 50% of the PGF2α, when they are treated with linoleic and linolenic acid (Zhangrui et al. 2005), and that the polyunsaturated fatty acids may also inhibit the synthesis of PGF2 a and hence induce a delay in luteolysis (Gulliver et al. 2012).
Table 1 lood serum concentration of Progesterone (ng ml-1) (± SD) and Cholesterol (mg/dl) (± SD) at the luteal phase in tropical hair sheep supplemented with corn oil at three levels on a dry matter basis.
| Treatments | |||
|---|---|---|---|
| Variable | 0% | 3% | 6% |
| Cholesterol | 102.14 ± 2.65b | 106.42 ± 3.48a | 108.57 ± 3.40a |
| Progesterone | 2.34 ± 0.66b | 3.31 ± 0.48a | 3.88 ± 0.24a |
SD: Standard deviation, a,b Means with different letters in the same row, indicate significant differences (p < 0.05).
The serum concentration of insulin (Table 2) was affected by the highest oil level (6%) included in the diet (p < 0.05) compared to the T0 and T3 concentrations. Mean levels of insulin (ng ml-1) in the present study are similar to those reported by Espinoza et al. (2008) when supplementing ewes with bovine fat (2.5 ± 0.11), a control diet (1.78 ± 0.11) and calcium soaps of fatty acids (1.72 ± 0.11). Herrera et al. (2003) found no differences between the levels of this hormone in sheep fed with 0 and 3% corn oil, a similar result of the one present work. Williams and Stanko, (2000) reported a higher insulin concentration in cows supplemented with polyunsaturated fatty acids and found that linoleic acid increases propionate production, which acts as a promoter of insulin secretion and prevents mobilization of adipose tissue fat. High concentrations of insulin reduce hepatic expression of progesterone catabolic enzymes such as cytochromes P450 2C and 3A (Lemley et al. 2008) and increases circulating progesterone concentrations (Moriel et al. 2008, Lopes et al. 2009).
Table 2 Insulin concentration in serum (mean ± standard deviation, ng ml-1), after estrus in tropical hair sheep supplemented with three levels of corn oil as percent of the diet on a dry matter basis
| Treatments | |||
|---|---|---|---|
| Time after estrus initiation (h) | 0% | 3% | 6% |
| 8 | 1.77 ± 0.30b | 1.81 ± 0.22b | 2.79 ± 0.20a |
| 11 | 1.76 ± 0.17b | 1.91 ± 0.23b | 2.13 ± 0.18a |
| 14 | 1.44 ± 0.57b | 1.67± 0.14b | 2.24 ± 0.15a |
| 17 | 1.49 ± 0.10b | 1.90 ± 0.13b | 2.49 ± 0.20a |
| 20 | 1.514 ± 0.131b | 1.943 ± 0.171b | 2.918 ± 0.134a |
SD: Standard deviation, a,b Means with different letters in the same row, indicates statistical differences (p < 0.05)
Estradiol concentrations (Table 3) in plasma were increased in sheep with supplemented corn oil treatments (p < 0.05) compared to the animals in the control group, and concentrations of this hormone became higher as ovulation approached. Robinson et al. (2002) reported an increase in blood serum estradiol in dairy cows supplemented with 18:3 and 18:2 fatty acids, compared with a control group. Similarly, Zachut et al. (2008) reported a greater estradiol concentration in plasma when cows were fed low and high levels of polyunsaturated fatty acids (4.9 and 5.1 pg ml-1) compared with the control group (3.5 pg ml-1). Concentrations of estradiol in blood plasma were positively related to oestrus length (Mondal et al. 2006), the diameter of the preovulatory follicle, and pregnancy rate in cows (Perry et al. 2014). Plasma levels of estradiol reported in this study could be attributed to an increase in ovarian steroidogenesis or possibly to decrease in steroid catabolism in the liver (Zachutl et al. 2011). The in vitro addition of C18: 3 n-3, present in the corn oil, increases the lifespan of progesterone and estradiol (Piccinato et al. 2010).
Table 3 Blood serum concentration of Estradiol (pg dl-1) (± SD) before, at and after estrus in tropical hair sheep supplemented with corn oil at three levels on a dry matter basis.
| Days to estrus presentation | Treatments (percent oil in the diet) | ||
|---|---|---|---|
| 0% (Control) | 3% | 6% | |
| -2 | 8.34 ± 0.82 b | 9.77 ± 0.68a | 9.27 ± 0.84a |
| 0 | 11.41 ± 1.36b | 13.53 ± 0.92a | 13.64 ± 0.48a |
| 2 | 10.71 ± 1.48 b | 12.81 ± 0.63a | 12.09 ± 0.63a |
SD: Standard deviation. Means with different superscript letters in the same row, are different (p < 0.05)
We conclude that inclusion of corn oil (3 or 6% of the diet) enhances blood serum concentrations of estradiol, progesterone, and cholesterol, meanwhile insulin concentration in serum was incremented with 6% of Corn oil in the diet, however, DWG may be compromised by corn oil added. This change promoted by corn oil in the diet over cholesterol, progesterone, estradiol and even insulin (at 6% oil inclusion level) could positively influence the reproductive traits in hair sheep in the tropics.











 text new page (beta)
text new page (beta)