Introduction
Livestock husbandry in the tropics is highly dependent on rainfall and air temperature fluctuations throughout the year (Calvosa et al. 2007), which lead to a forage deficit in the dry and winter seasons (Enríquez et al. 2003), during which grasses have low nutritional values e. gr. low concentrations of crude protein and digestible dry matter. Tropical leguminous forage trees represent an alternative food that can provide nutrients to the rumen microorganisms and can increase voluntary intake. Some leguminous species are a source of high quality proteins and are easily adaptable to different soil and climatic conditions (Sprent et al. 2010). The main attributes that tropical legumes possess in order to incorporate them into cattle production units are: 1) High protein content (14 - 28%); 2) Low fiber concentration (< 40%); 3) Good acceptance by animals; and 4) Increase in production parameters (Valles-de la Mora et al. 2016).
The tropical forage legume Cratylia argentea was selected because of its nutritional value (CP < 23%, in vitro dry matter digestibility between 40 - 55%) and moderate concentration of tannins and other secondary metabolites (Lascano and Plazas 2003). Indeed, this legume has shown an in vitro anthelmintic effect against gastrointestinal nematodes (Von son-de Fernex et al. 2012). It is always necessary to generate information on the effect of including a nutritious legume in low quality grass basal diets, upon intake and digestibility of the whole diet. Thus the objective of this research was to estimate the intake, digestibility, and nitrogen balance of F1 steers (Hostein - Zebu) fed with different mixtures of the tropical shrub legume Cratylia argentea and the grass Brachiaria arrecta.
Materials and methods
Experimental site
The study was conducted in the municipality of Atzalan, Veracruz, Mexico (20° 02' North and 97° 06' West), 114 masl, in a hot-humid climate (Af(m)w’(e)), with an average daily temperature of 23.9 ± 0.5 °C, and rainfall of 1 931 ± 334 mm. Four F1 steers (Holstein x Zebu) with an average live weight of 214.5 ± 9.3 kg, were used from april 8 to june 17, 2013. The steers were allocated in individual pens in order to facilitate sample collection. The animals were allowed to get used to daily management for 30 d previous to the beginning of the experiment. Animáis were dewormed (levamisol, 7.5 mg kg-1 LW, intramuscular; albendazol, 8.5 mg kg-1 animal-1), and mineral salt (50 g·animal-1·d-1) and water ad libitum were also supplied.
A Latin square (4 x 4) design was used, considering four periods of 17 d, each with a 10 d adaptation period and a 7 d measurement period. Ten days was enough time for the disappearance of the effect of some secondary metabolites in the previous treatment, as suggested by the work of Woodward and Reed (1997) and de Oliveira et al. (2007). Besides, because of the high turnover rates of the microbial population, it is expected that the microbial ecosystem and with it protein and carbohydrate degradation patterns, reach a steady State after 9 d irrespective of the previous treatment (Ahnert et al. 2015).
The experimental diets were combinations of Cratylia argentea and Tanner grass (Brachiaria arrecta) hay, both in % in the mixture, to add up to 100%, as follows: (T1) 0:100; (T2) 15:85; (T3) 30:70; and (T4) 45:55. During the experimental period, steers were fed with an additional 10% of the intake shown during the adaptation period (5% C. argentea + 95% Tanner grass hay). Steers were weighted, after a 17 h fast, at the beginning and end of each period.
Sample collecting and processing
Samples were taken daily at the same time during the seven days of the measurement period. The quality of the offered and residual forage was analyzed in 300 g samples. Five-ml blood samples were taken from the caudal vein of each animal using vacutainer tubes without anticoagulant, in order to determine the concentration of urea in blood serum (BUN). Those samples were taken at feeding time (8:00) and six hours after. Fecal collection was also performed individually on the measurement period; samples were collected immediately after the animal defecated in order to avoid losses by trampling. From the total fecal samples of each animal, a sub-sample of 500 g was placed in polyethylene bags and transported to the laboratory to be analyzed for dry matter and nitrogen.
For total urine collection, an apron was designed to cover the prepuce with the aid of harnesses, and through a funnel and a hose, it allowed the urine to accumulate in a plástic container, which was poured every two hours into a second container. Total urine was measured every 24 h. A daily sample of 200 mi of urine was collected and acidified with sulfuric acid (pH = 2) in order to prevent bacterial growth. Samples were frozen at -20 °C until analysis. Daily water consumption was measured by difference. Every day, the residual forage was weighed at 7 h, as well as, residual water, total urine excretion, urine pH, and fecal excretion. Then, new forage on offer was placed in the feedthrough at 16 h.
Chemical analysis
Samples of forage on offer and orts (residual forage) as well as feces were analyzed following the AOAC (1995) methods for dry matter (DM), organic matter (OM) and crude protein (CP, 6.25 x N). Samples were also analyzed for neutral detergent fiber (NDF), acid detergent fiber (ADF) and lignin by solubilization of cellulose with 72% sulfuric acid (Van Soest et al. 1991) using an ANKOM 200 Fiber Analyzer unit (Ankom Technology Corp. Macedon, NY, USA). Dry matter (DM) intake (kg DM.animal-1·d-1) was the difference between feed on-offer and orts, after 24 h (Mupeyo et al. 2011).
Blood urea nitrogen, urine urea N, total feces and urine, and purine derivatives
The determination of the levels of blood urea nitrogen (BUN, mg dL-1), were done with the IDEXX VetTest® blood chemistry analyzer (USA) that uses dry chemistry. Urine urea-N (mg dL-1) was obtained by the Ultra-Violet Enzyme kinetic test. The total amount of feces (g·animal-1·d-1) and urine (L·animal-1·d-1) were measured. To determine uric acid and allantoin (purine derivatives) in urine, a kit of Spinreact analysis was used for the former; whereas for the latter, the colorimetric technique of Chen and Orskov (2003) was used, as well as the equations to calculate microbial nitrogen supply. Total N excreted in urine (UN, g d-1) was calculated from urinary urea-N excretion (UUN, g d-1) using the equation: UN = 63.9 + 0.899*(UUN) that is the average of the two linear relationships presented by Spek et al. (2013).
Experimental design and statistical analysis
The experimental design was a balanced 4x4 latin-square with periods as rows and sequences as columns, with animals assigned randomly to a sequence. In a first step, an ANOVA with PROC MIXED of SAS (SAS, 2013), using the effects of period and treatment as fixed, was performed in order to select according to the BIC statistic, the best covariance structure among simple, compound symmetry (CS), auto regressive (AR(1)), autoregressive with the animal as random effect (AR(1)+RE), Toeplitz (TOEP) and unstructured (UN). The best covariance structure was (AR(1)+RE). A second analysis of variance was conducted in order to request linear, quadratic and cubic contrasts of treatments, as well as least squares treatment means. The cubic effect of treatment was not significant on any variable and for this reason it is not presented in the tables. After these analyses, first and second order polynomials were fit to the response variables least squares means and their standard errors, and the corresponding treatment levels, in order to help in the interpretation of the previous tendency analyses.
Results
Results are presented in Tables showing treatment least squares means and their common standard error, along with the level of significance of the linear and quadratic contrasts. Unless otherwise said, probability levels are quoted only in tables, in order to avoid them being unnecessarily repeated in the text. The ANOVA for live-weight (kg animal-1) did not show any significant effect of the period, or of the treatment contrasts: Linear (p = 0.5036), quadratic (p = 0.7911) or cubic (p = 0.9332). Live-weight means for treatments 0:100, 15:85, 30:70 and 45:55 were: 214.8, 214.9, 214.4 and 213.9 kg, respectively, with an experimental standard error of 9.3 kg. Since live-weight was not affected by periods and treatments, intake results are presented on an animal basis, taking into consideration that expressing them on a live-weight or metabolic weight basis does not alter the results of the statistical analysis and their interpretation.
Table 1 shows the Chemical composition of the grass and legume used in the study. The OM, NDF and ADF of the grass hay were significantly larger than the foliage of the legume. Opposite to that, the concentrations of ash, CP and ADL of the legume were significantly larger than those of the grass. The two largest differences were those due to CP and ADL, as legume foliage was 3.1 and 1.9 times larger in CP and ADL, respectively, than grass hay. Table 2 shows the effect of increasing the proportion of Cratylia argentea foliage in the diet, on the Chemical composition of the feed on offer, orts and feces. The linear effect of the treatment was significant or highly significant upon the concentrations all Chemical components, except orts-CP and feces-NDF in which it was not significant. The quadratic effect of treatment was significant only on orts-CP. In the case of CP the trend was to increase linearly on feed on offer and feces, showing only a quadratic trend (p < 0.05) on orts. Conversely, the linear trend was highly significantly negative on NDF and ADF, except on feces NDF. In the case of ADL, its concentration increased highly significantly on feed on offer, orts and feces as C. argentea proportion in the diet increased.
Table 1 Chemical composition (%, mean ± standard error) of diet components Brachiaria arrecta and Cratylia argentea, offered to stalled F1 (Holstein x Zebu) bullocks.
| Chemical component | Diet component (g kg-1 DM) | |
|---|---|---|
| Brachiaria arrecta | Cratylia argentea | |
| Organic matter (OM) | 91.2 ± 3a | 88.8 ± 1b |
| Ash | 8.8 ± 3a | 11.2 ± 1b |
| Crude protein (CP) | 6.2 ± 3a | 19.3 ± 6b |
| Neutral detergent fiber (NDF) | 81.2 ± 1a | 64.9 ± 2b |
| Acid detergent fiber (ADF) | 47.3 ± 6a | 41.4 ± 2b |
| Acid detergent lignin (ADL) | 10.6 ± 3a | 20.1 ± 1b |
Different letters for the components of the diet indicate statistical differences (p < 0.05).
Table 2 Effect of increasing the level of inclusion (%) of Cratylia argentea foliage on a basal diet of Brachiaria arrecta on crude protein and cell wall fiber components of forage offered, orts and excreted feces by stalled bullocks.
| Response variable | Cratylia argentea: Brachiaria arrecta (%) | S.E. | Contrast, P > F | ||||
|---|---|---|---|---|---|---|---|
| 0:100 | 15:85 | 30:70 | 45:55 | Linear | Quadratic | ||
| Crude protein (CP, %) | |||||||
| On offer | 6.21 | 8.18 | 10.14 | 12.11 | 0.15 | <0.0001 | 1.0000 |
| Orts | 8.67 | 9.87 | 10.18 | 8.11 | 0.47 | 0.5247 | 0.0136 |
| Feces | 5.98 | 6.54 | 6.76 | 7.15 | 0.28 | 0.0136 | 0.5073 |
| Neutral Detergent Fiber (NDF.%) | |||||||
| On offer | 80.98 | 78.55 | 76.13 | 73.70 | 0.25 | <0.0001 | 1.0000 |
| Orts | 81.13 | 77.41 | 73.31 | 70.68 | 1.23 | 0.0002 | 0.5000 |
| Feces | 70.03 | 70.45 | 70.75 | 71.53 | 0.70 | 0.1834 | 0.7655 |
| Acid Detergent Fiber (ADF, %) | |||||||
| On offer | 47.28 | 46.33 | 45.43 | 44.55 | 0.53 | 0.0030 | 0.9370 |
| Orts | 48.90 | 48.15 | 46.90 | 45.53 | 0.59 | 0.0036 | 0.4485 |
| Feces | 43.23 | 44.00 | 45.25 | 46.93 | 0.60 | 0.0044 | 0.4549 |
| Acid Detergent Lignin (ADL, %) | |||||||
| On offer | 10.60 | 11.98 | 13.40 | 14.83 | 0.29 | <0.0001 | 0.9253 |
| Orts | 13.05 | 15.23 | 16.70 | 18.38 | 0.43 | <0.0001 | 0.5796 |
| Feces | 15.20 | 16.77 | 17.92 | 19.17 | 0.52 | 0.0009 | 0.7747 |
Table 3 shows the effect of treatment on daily intake and apparent digestibility of DM, CP, NDF, ADF and ADL. The linear effect of treatment was significant or highly significant on most variables, except intake of NDF and intake and apparent digestibility of ADF. The quadratic effect was significant only on apparent digestibility of DM and NDF, but showed a strong tendency to significance (p < 0.10) on digestibility of CP, ADF and ADL. Overall, in the present study, increasing the level of inclusion of foliage of the shrub legume Cratylia argentea in a low quality Tannergrass hay-based diet, increased intake, as well as the apparent digestibility of the Chemical components of the diet. Table 4 shows the effect of treatment on BUN, and urine volume, pH and urea-N, N-balance and its components and purine derivatives. The linear effect of treatment was highly significant on both BUN at feeding time and six-hours after feeding and on urine pH, while the same effect was significant on urine urea-N and not significant on urine volume. The quadratic effect of treatment was highly significant on BUN six-hour after feeding and urine pH. With respect to N balance and its components (Table 4), the linear effect of the C. argentea level was highly significant on N-intake, N-output from urine and N-balance and not significant upon output of N from feces. On the other hand, the quadratic effect of C. argentea content in the diet was not significant on N-balance and its components. Only the linear effect of treatment was significant on daily uric acid production, but not on allantoin and purine derivatives, and neither was its quadratic effect significant on any of these three variables.
Table 3 Effect of increasing the level of inclusion (%) of Cratylia argentea foliage on a basal diet of Brachiaria arrecta hay, on intake and apparent digestibility by stalled bullocks.
| Response variable | Cratylia argentea: Brachiaria arrecta (%) | S.E. | Contrast, P > F | ||||
|---|---|---|---|---|---|---|---|
| 0:100 | 15:85 | 30:70 | 45:55 | Linear | Quadratic | ||
| Dry matter (DM) | |||||||
| Intake (g animal-1) | 6 310 | 7 026 | 8 114 | 8 374 | 730 | 0.0278 | 0.7361 |
| Intake (g kg-1 LW0:75) | 113.66 | 124.45 | 144.98 | 149.38 | 12.65 | 0.0316 | 0.7954 |
| Apparent digestibility (%) | 65.38 | 65.63 | 67.50 | 73.45 | 1.10 | 0.0004 | 0.0198 |
| Crude protein (CP) | |||||||
| Intake (g animal-1) | 334 | 552 | 808 | 1112 | 83 | 0.0001 | 0.5731 |
| Intake (g kg-1 LW0:75) | 6.01 | 9.88 | 14.29 | 19.66 | 1.16 | <0.0001 | 0.5253 |
| Apparent digestibility (%) | 60.06 | 73.30 | 79.29 | 85.97 | 2.45 | <0.0001 | 0.0729 |
| Neutral detergent fiber (NDF) | |||||||
| Intake (g animal-1) | 5 143 | 5 562 | 6 301 | 6 330 | 587 | 0.0696 | 0.7045 |
| Intake (g kg-1 LW0:75) | 92.74 | 98.33 | 112.61 | 112.74 | 9.97 | 0.0791 | 0.7727 |
| Apparent digestibility (%) | 69.66 | 68.92 | 69.77 | 73.91 | 1.06 | 0.0083 | 0.0029 |
| Acid detergent fiber (ADF) | |||||||
| Intake (g animal-1) | 2 931 | 3 227 | 3 678 | 3 761 | 562 | 0.1093 | 0.7702 |
| Intake (g kg-1 LW0:75) | 72.24 | 70.11 | 84.17 | 89.24 | 11.89 | 0.1155 | 0.6646 |
| Apparent digestibility (%) | 67.96 | 66.88 | 67.17 | 71.13 | 5.20 | 0.0926 | 0.0617 |
| Acid detergent lign in (ADL) | |||||||
| Intake (g animal-1) | 610 | 798 | 1 036 | 1 183 | 120 | 0.0048 | 0.8618 |
| Intake (g kg-1 LW0:75) | 11.00 | 14.12 | 18.49 | 21.04 | 2.00 | 0.0041 | 0.8908 |
| Apparent digestibility (%) | 46.12 | 48.24 | 52.47 | 61.33 | 1.47 | 0.0003 | 0.0502 |
Table 4 Effect of increasing the level of inclusion (%) of Cratylia argentea foliage on a basal diet of Tanner grass (Brachiaria arrecta) hay, on blood urea nitrogen and other urine nitrogen-related variables, and nitrogen balance of stalled bullocks.
| Response variable | Cratylia argentea: Brachiaria arrecta (%) | S.E. | Contrast, P > F | ||||
|---|---|---|---|---|---|---|---|
| 0:100 | 15:85 | 30:70 | 45:55 | Linear | Quadratic | ||
| Blood Urea Nitrogen (BUN) and other urine variables | |||||||
| BUN at feeding time (mg dL-1) | 2.75 | 5.50 | 10.25 | 12.25 | 0.62 | <0.0001 | 0.2954 |
| BUN six h after feeding (mg dL-1) | 3.50 | 8.00 | 12.00 | 14.75 | 0.79 | <0.0001 | 0.0014 |
| Urine volume (L d-1) | 4.15 | 4.65 | 5.28 | 6.08 | 1.23 | 0.0549 | 0.6795 |
| Urine pH | 7.85 | 8.35 | 8.53 | 8.53 | 0.06 | <0.0001 | 0.0036 |
| Urine urea-N (mg dL-1) | 181.58 | 241.65 | 359.50 | 437.55 | 54.85 | 0.0123 | 0.8718 |
| Nitrogen balance | |||||||
| Intake (g·animal-1 ·d-1) | 53.50 | 88.36 | 129.22 | 177.86 | 13.28 | 0.0001 | 0.5731 |
| Feces (g·animal-1 ·d-1) | 20.64 | 23.64 | 25.42 | 24.55 | 1.94 | 0.0832 | 0.2754 |
| Urine (g·animal-1 ·d-1) | 71.30 | 73.10 | 79.73 | 84.80 | 2.24 | 0.0009 | 0.4278 |
| Balance (g·animal-1 ·d-1) | -38.44 | -8.38 | 24.07 | 68.51 | 11.29 | 0.0001 | 0.4730 |
| Purine derivatives (PD) in urine | |||||||
| Uric acid (mmol d-1) | 0.12 | 0.38 | 0.85 | 0.89 | 0.21 | 0.0137 | 0.5943 |
| Allantoin (mmol d-1) | 3.80 | 4.76 | 5.35 | 7.33 | 1.96 | 0.1851 | 0.6610 |
| Purine derivatives (mmol d-1) | 3.92 | 5.13 | 6.20 | 8.21 | 2.07 | 0.1274 | 0.7608 |
Table 5 presents the effect of increasing C. argentea proportion in the diet on daily intake and apparent digestibility of DM, CP, NDF, ADF and ADL, in the form of a linear or quadratic function. With the exception of CP intake, the intakes of the remaining Chemical components showed a weak relationship with treatment, as indicated by R2-values below 0.52 and as low as 0.01. DM intake increased linearly by 48.5 g animal-1 per each percent unit of C. argentea inclusion in the diet. In the case of CP, the increase was 17.3 g animal-1 per percent unit of the legume, while for ADF intake and digestibility the lines were practically flat. The negative coefficient in the quadratic regression indicated that the prediction line reached a minimum value of digestibility at 8.4, 14.1 and 0.3% of Cratylia argentea in the diet for DM, NDF and ADL, respectively. After that point, the digestibility of DM, NDF and ADL increased at an increasing rate in response to C. argentea level in the diet. Table 5 also presents the effect of increasing C. argentea proportion in the diet on urine variables, nitrogen balance and purine derivatives, also as linear or quadratic functions. The positive linear effect of the treatment was highly significant on BUN at feeding time and 6 h after feeding, and urine pH, whilst there was a strong tendency to be significant on urine volume. BUN six hours after feeding and urine pH were significantly affected by the quadratic effect of treatment. The intercepts of the regression lines for BUN at feeding time and six hours after feeding were significantly different (p < 0.05) between them, indicating that the BUN content increased from feeding to six hours after by 1.2 mg dl-1. On the other hand, the slopes of the lines were not significantly different (p > 0.05) between them: 0.222 vs 0.252 mg dL-1 per percent unit of legume increase in the diet.
Table 5 Equations resulting from fitting linear and quadratic regression functions in order to depict relationships between the level of inclusion (%) of Cratylia argentea foliage (x) on a basal diet of Tanner grass (Brachiaria arrecta) hay, on several response variables (y).
| Response variable | Regression equation parameters1 | R2 | Sy.x 2 | ||
|---|---|---|---|---|---|
| a | B | c | |||
| Feed | |||||
| Intake (g.bullock-1.d-1) | |||||
| Dry matter | 6 364 | 48.53 | ---------- | 0.2890 | 1365 |
| Crude protein | 313.0 | 17.27 | ---------- | 0.7987 | 155.4 |
| Neutral detergent fiber | 5 189 | 28.67 | ---------- | 0.1795 | 1099 |
| Acid detergent fiber | 2 958 | 19.61 | ---------- | 0.1018 | 1044 |
| Acid detergent lignin | 613.2 | 13.05 | ---------- | ||
| Digestibility (%) | |||||
| Dry matter | 65.50 | -0.1111 | 0.006333 | 0.7397 | 2.136 |
| Crude protein | 62.10 | 0.5581 | ---------- | 0.8031 | 4.955 |
| Neutral detergent fiber | 69.75 | -0.1533 | 0.005422 | 0.5273 | 2.048 |
| Acid detergent fiber | 66.82 | 0.06533 | ---------- | 0.0143 | 9.726 |
| Acid detergent lignin | 46.25 | -0.0046 | 0.007489 | 0.8379 | 2.842 |
| Blood and urine | |||||
| BUN at feeding time (mg dL-1) | 2.700 | 0.2217 | ---------- | 0.9038 | 1.296 |
| BUN six h after feeding (mg dL-1) | 3.463 | 0.3392 | -0.001944 | 0.9055 | 1.521 |
| Urine volume (L d-1) | 4.077 | 0.0428 | ---------- | 0.1018 | 2.279 |
| Urine pH | 7.857 | 0.0398 | -0.000556 | 0.8749 | 0.1166 |
| Urine urea-N (mg dL-1) | 172.2 | 5.905 | ---------- | 0.5169 | 102.3 |
| N-Balance (g.bullock-1.d-1). | |||||
| Intake | 50.14 | 2.76 | ---------- | 0.7984 | 24.86 |
| Feces | 21.54 | 0.09007 | ---------- | 0.1570 | 3.742 |
| Urine | 70.16 | 0.3142 | ---------- | 0.6311 | 4.307 |
| Balance | -41.56 | 2.355 | ---------- | 0.7974 | 21.29 |
| Purine derivatives (mmol L-1) | |||||
| Allantoin | 3.633 | 0.07453 | ---------- | 0.1184 | 3.646 |
| Uric acid | 0.1430 | 0.01853 | ---------- | 0.4075 | 0.4006 |
| Purine derivatives | 3.774 | 0.09293 | ---------- | 0.1584 | 3.841 |
1The regression function is: y = a +bx + cx2, where: a is the intercept (value of y when x = 0), b is the linear coefficient of regression (increase in y units per unit of increase in x), and c is the quadratic coefficient of regression (increase or decrease in y units per square unit of increase in x); if c is positive, the dependent variable has a minimum, otherwise it has a maximum. 2Sy:x is the standard deviation of the residuals.
The linear effect of treatment was highly significant on N-intake, urine output and apparent N-balance, while the same effect showed a tendency (p < 0.10) to have a significant effect on N output from feces. The quadratic effect of treatment did not affect N-balance variables. It was relevant that N-balance was negative at 0:100 and 15:85 treatments and positive at higher levels of inclusion of C. argentea in the diet. While the general trend for purine derivatives was to increase as the percent of C. argentea increased in the diet, only the linear effect of treatment was significant on uric acid. Neither the linear nor the quadratic effects of treatments were significant on allantoin or purine derivatives (Table 4).
Urine volume response to C. argentea in the diet was linearly positive, but not significant, as indicated by the reduced slope of the regression line: 0.043 L animal-1 per percent unit of increase legume content. Undoubtedly the high variation within treatments contributed to the lack of significance. The urine pH response to treatment was quadratic, with a predicted máximum pH value of 8.57 at a legume content of the diet of 35.8%. The urine urea nitrogen responded to treatment in a linear and positive way, with an increase of 5.91 mg dL-1 per unit of increase in the legume content of the diet. Table 5 shows the linear regression models fit to the data and the resulting prediction lines for nitrogen balance and its components. In all cases, the response to the treatments was highly significantly linear and positive, except with feces N output that was not significant. Predicted values at the mid-point legume content in the diet of 22.5% were 23.57 g N animal-1 d-1 and 77.13 g N animal-1d-1 for feces and urine, respectively, which indicate that the former contributed with around 23% and the latter with 77% of the average daily output of nitrogen. Making the regression equation for balance equal to zero and solving for %Ca, gave a value of 17.61% which is the percent C. argentea in the diet to yield a zero N balance.
Discussion
The experimental diets consisted of four proportional combinations of C. argentea with B. arrecta hay to add up to 100% (0:100, 15:85, 30:70 and 45:55). The Chemical composition of either species represents the typical values reported for these tropical plants. Tanner grass CP content (6.2 ± 1.6%) and digestibility (53.2 ± 5%) were below the optimal, and this was associated to the advanced age of Tanner grass hay of the present research, that was greater than 90 days. It has been widely documented that grasses harvested at an early age have better quality than mature ones (Arthington and Brown 2005). For C. argentea, CP values, NDF and ADF were consistent with those reported by González-Arcia et al. (2012) and Castillo et al. (2013), under similar climate and soil conditions. However, its lignin content of 20.1 ± 7.9% was higher than the ones reported by the same authors: 15.1 and 16.7%, respectively. With such a high lignin content, a decrease in digestibility was expected, due to its negative correlation with digestibility; furthermore, lignin content is positively correlated with plant maturity (Jung and Alien 1995). However, lignin is a structural polymer of phenolic nature, which quantification by the technique of Van Soest can be overestimated in the presence of tannins and other polyphenolic compounds (Aufrére and Guérin 1996). Although C. argentea is considered a free-tannin legume (Bernal et al. 2008), a moderate concentration of polyphenols with high biological activity has been reported, which can lead to lignin content overestimation.
The beneficial effect of adding increasing amounts of highly nutritious legumes to a low quality tropical grass diet has been known for some time. In their classical investigation, Minson and Milford (1967) added Luceme (Medicago sativa) to the basal diet of pangolagrass (Digitaria decumbens) given to rams in 100 g incremental amounts from 0 to 300 g ram-1 d-1, and found that both intake and apparent digestibility increased linearly with Lucerne level.
In the present experiment, increasing the proportion of C. argentea in the diet increased significantly DM and CP intake and apparent digestibility (Tables 3 and 5). The information that exists on the intake of C. argentea by cattle, agrees in that the inclusion of this legume in the diet increases the DM intake. Sánchez and Ledin (2006) compared the voluntary DMI between groups of dairy cows fed sorghum silage, with and without the addition of C. argentea (2 and 3 kg DM) and found that supplemented treatments had higher DM intake than non-supplemented ones. Hess et al. (2004) also found that DM intake by 35.5 kg of LW lambs, increased in a highly significant manner as levels of C. argentea in the diet increased, but conversely, apparent digestibility of OM highly significantly decreased as the level of C. argentea increased.
Intake and apparent digestibility of CP increased highly significantly when the level of C. argentea increased. Benavides et al. (2010) found that when this legume was included in the diet, an increase in the voluntary intake of C. argentea occurred and that this fact was associated with the use of fresh leaves. Cratylia argentea has a high CP content of around 20% (Castillo et al. 2013). In this study, the increase in the replacement rate of the grass by the legume led to a greater availability of the forage on offer and a higher quality as well, that resulted in a significant increase of CP intake with the increase of C. argentea in the diet. In the present experiment animals ingesting Tanner grass hay, low in CP, resulted in the lowest DM and CP intake. In general, DM and nutrient intake of low quality tropical grasses benefit from adding a nitrogen source to the diet. Coleman and Moore (2003) related forage CP content (g kg-1 DM) and DM intake (g kg-1 LW) using the nonlinear equation DMI = 23.5 - 38.5e-0:032*CP and found that when dietary CP was below 8%, the relationship was strongly positive because it is not until the minimum N requirements of the rumen microbes are met that fiber digestion becomes more efficient, undigested forage does not accumulate in the rumen, passage rate increases and so it does DM intake. Then, as the ruminal requirement of N is met and deficiency overcame, the relationship becomes weak or almost nil. In the present case, the diet on offer CP contents were lower than 8% only in treatment T1 (without Cratylia argentea).
Due to their high nutrient content, tropical legumes can be an alternative tool to improve the diet of grazing cattle. Nevertheless, as noted by Mueller-Harvey (2006), these plants can have negative effects on ruminants because of the presence of secondary compounds and lignin, of which they may contain high levels. Fiber components like lignin can limit DM intake in cattle, but at low contents those secondary compounds may help to modulate ingestion by ruminants (Sandoval-Castro et al. 2005), like in situations when some nutrients are present in excess, or when there is an imbalance of nutrients (low energy or the presence of toxins), which limits the intake through a satiety mechanism (Provenza 2006). In this study, it appears that DMI was not affected by any of the above factors. C. argentea has been classified as a legume free of tannins with low levels of secondary compounds. However, qualitative studies of thin layer chromatography, revealed incipient levels of flavonol glycosides, flavones, chlorogenic acid, terpenes and saponins that do not affect the intake, but instead, they may have some beneficial effects (von Son-de Fernex et al. 2016). According to Poppi and Mc Lennan (1995) losses of protein or its incomplete net transfer to the lower tract will occur when CP content exceeds approximately 210 g of CP kg-1 of digestible OM (DOM), this ratio represents a degradable CP/available energy relationship for ruminal microbes, and can be used to identify situations in which significant losses of ingested protein might occur. Tropical grasses range in digestibility from approximately 55 to 65% and are unlikely to exceed the critical CP/DOM ratio, but many tropical legumes exceed this value and losses may be expected. It is noticeable that the high CP/DOM values where losses occur are generally seen in the grazing situation or where fresh pasture is consumed and there is highly soluble protein from fresh leaf intake. Poppi and McLennan (1995) stated that if legumes are able to increase DM intake by 30%, they could provide enough protein to the intestine to increase milk production by 2.6 kg cow-1 d-1. Castillo-Gallegos et al. (2014) studied the CP and DOM contents of the esophageal extrusa of Holstein-Zebu cows that grazed a native grass-based pasture in monoculture (NP) or associated with the herbaceous legume Arachis pintoi (NPA), using a rotation of one day of grazing per 20 d of recovery that produced a forage with high digestibility of OM and high CP content, as well as low DM content. The NP and NPA treatments showed 165 and 223 g CP kg-1 DOM, respectively, suggesting that the association incurred in net losses of CP transfer, due to an energy deficiency in the rumen.
In the present investigation, the increasing amounts of inclusion of C. argentea to the diet, led to increases in OM intake and apparent digestibility. The CP/DOM ratios were: 130, 122, 153 and 171 g CP kg-1 DOM, for treatments 0:100, 15:85, 30:70 and 45:55, respectively. Thus, none of the four treatments would have incurred in losses of protein or incomplete net transfer as they never reached the 210 g of CP kg 1 of DOM threshold. This is indeed a very attractive situation from an economical point of view since the efficient CP/DOM balance would make unnecessary the use of costly energy sources in agrosilvopastoral Systems where Cratylia argentea is one of the main feeds for browsing/grazing ruminants.
The increment on CP intake and apparent digestibility due to the incremental proportions of C. argentea in the diet, was associated with a linear increase in BUN at feeding time and six hours after, and with increases in urine urea N as well, being the correlation coefficients between CP intake and BUN0, BUN6 and UUN: 0.7943 (p < 0.01), 0.7585 (p < 0.01) and 0.5921 (p < 0.05), respectively. Gonzalez-Arcia et al. (2012) found that BUN concentration in Holstein x Zebu heifers grazing B. brizantha as monoculture was 7.2 ± 0.2 mg dL-1, whereas those heifers on the same grass but associated with C. argentea, had a BUN concentration of 10.8 ± 0.6 mg dL-1, being the difference significant (p < 0.05). The significant (p < 0.05) association between CP intake and BUN levels in cattle fed with C. argentea, opens the possibility of using BUN measurements to determine the CP intake and the proportion of legume ingested, by animals under grazing/browsing conditions, where C. argentea is a component of the vegetation. However, controlled field studies are needed in order to confirm this hypothesis.
The normal BUN concentration in healthy animals ranges from 8 to 24 mg dL-1 (Roa-Vega et al. 2017). It has been reported that cows with BUN concentration higher than 20 mg dL-1 show poor conception rates (Ferguson et al. 1993). In the present experiment, the BUN values at feeding time of treatments 0:100 and 15:85 were below this ample range, while BUN values six hours after feeding were below only on treatment 0:100 (Table 4). The maximum BUN mean value was 14.8 mg dL-1 for treatment 45:55 six hours after feeding, which was about the middle of the normal reference values. Roa-Vega et al. (2017) tested four treatments: Grazing of Brachiaria decumbens without probiotic (T1) and with probiotic (T2), like T1 and T2 but supplemented with 3.5 kg·head-1 ·d-1 of C. argentea (T3 and T4) and found BUN values of 15.9, 22.2, 22.8 and 23.6 mg dL-1, respectively, values that were by far superior to the ones found in the present experiment. Our results highlight the importance of choosing an average time after feeding that be representative of the normal baseline circulating levels of BUN. Perhaps the 24 h period after feeding utilized in this experiment would be the most appropriate, but this needs future confirmation by taking samples at regular intervals after feeding.
Urine volume increased with increasing proportions of C. argentea in the diet (Table 4), although non-significantly (p = 0.0549). It has been reported that saponins have a diuretic effect on cattle (Soetan et al. 2014). Qualitative studies of thin layer chromatography have revealed low levels of saponins in C. argentea foliage (von Son-de Fernex et al. 2016) that might have stimulated urine output on those animals ingesting the legume. The normal value for urine pH is around eight. Kume et al. (2011) reported that there were positive correlations between urine pH and urinary K concentration or K intake in dairy cows. Kamiya et al. (2017) gave increasing levels of sweet potato condensed distillers solubles (SCDS: 0, 10, 20 and 30%), a feed having high K concentration, to Japanese black steers and found that both intake of K and urine pH presented a significant (p < 0.05) increasing linear response to SCDS levels. Tiemann et al. (2009) found that C. argentea mean concentration of 23.91 mg kg-1 DM was significantly (p < 0.05) higher than those of Desmodium velotinum, Calliandra calothyrsus, Flemingia macrophylla and Leucaena leucocephala by 8.16, 15.05, 13.29 and 6.21 mg kg-1 DM, respectively. Therefore, being C. argentea a legume with a high K concentration, it was feasible that in the present experiment, K intake would have increased with increasing levels of the legume in the diet, leading to higher values of urine pH. Besides, urine urea N also increased significantly (p < 0.05) in response to the increasing levels of C. argentea (Table 4), and that also contributed to urine alkalization and pH rise in urine.
The three components of N balance showed a linear increase as C. argentea proportion increased in the diet. However, the slope of the lines were practically flat for feces (0.09 g N/%-unit of C. argentea in the diet) and urine (0.31 g N/%-unit of C. argentea in the diet) (Table 5; Figure 1). Hess et al. (2004) fed sheep with a basal diet of B. dictyoneura grass hay, with and without the inclusion of C. argentea (67:33 or 33:67), and showed that the addition of the legume to the diet increased DM intake, N intake, as well as N urinary excretion, and also, that the increased N output was related to a greater urine volume and higher water intake. The former is consistent with the results of the present investigation, where the total amount of urine increased in response to increments of C. argentea (p = 0.0549; Tables 4 and 5), but it did not agree on water intake, as in the present trial, the effect of treatment was not significant (p = 0.2500) on this variable, that showed a mean and standard error of 17.7 ± 1.1 L animal-1 d-1 of water.
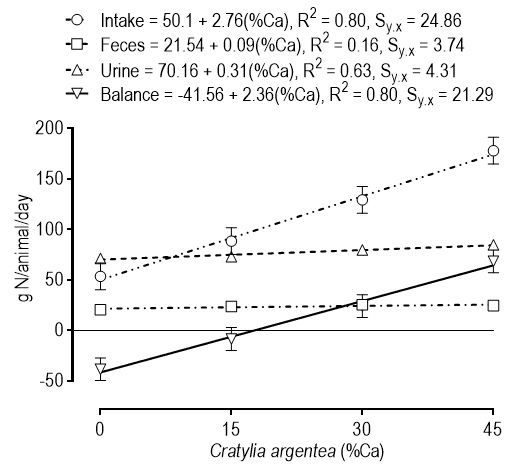
Figure 1 Relationships between the proportion of Cratylia argentea in the basal diet of Tanner grass (Brachiaria arrecta) (0:100, 15:85, 30:70 and 45:55), on nitrogen balance of F1 (Holstein x Zebu) bullocks fed under stalled conditions. Dots are least squares means and vertical bars their standard errors. The S y:x is the standard deviation of the residuals in units of y.
At the end, N-balance was negative for treatments 0:100 and 15:85 of C. argentea: B. arrecta. If the linear equation for N-balance (Table 5) is equaled to zero and solved for %Ca, it results in a value of 17.61%, which is the proportion of C. argentea on offer to attain zero nitrogen balance (Figure 1). The immediate implication of this fact is that the grass alone could not support even maintenance requirements of CP for the type of cattle used in the experiment. Thus, this legume can be a useful tool to improve cattle nutrition, only if it is offered in proportions higher than 30% of a basal diet of low quality grass.
Purine derivatives in urine, uric acid and allantoin, did not show differences between treatments (p > 0.05). In contrast, Hess et al. (2004) mentioned an increase in the excretion of allantoin and purine derivatives, on sheep fed with Cratylia, suggesting that the provided metabolic protein improved due to an increase in the contribution of microbial protein. In the present investigation, it was not possible to estimate the microbial protein synthesis from the equations used by Chen and Orskov (2003), since these resulted in negative values. Dragomir et al. (2007) using the same method, were unable to determine the microbial protein synthesis in sheep, and explained that this happened because the equation parameters were estimated for animals that had higher food intakes and consequently, when the values generated fall outside the intake range, it is not possible to estimate the microbial protein synthesis. Also, these authors mentioned that they probably did not get the expected values of protein synthesis, because the conversion coefficients of the energy available in the rumen and nitrogen, necessary for producing the microbial protein, were highly susceptible to plant maturity and conservation method.
Conclusions
Four conclusions could be derived from the present experiment: Increasing the proportion of the shrub legume Cratylia argentea in the basal diet of Tannergrass (Brachiaria arrecta), improved intake and digestibility of DM and its Chemical components; The significant correlation between BUN levels and CP intake in cattle fed with C. argentea, opens the possibility of using BUN to predict CP intake and the proportion of legume ingested by animals under grazing/browsing conditions, where C. argentea is a component of the vegetation, providing previous confirmation by controlled field measurements; Since the C. argentea levels of 0 and 15% lead to negative N-balances, then, a minimum of 30% of the legume foliage in the diet must be offered to obtain a positive balance of nitrogen; And the four C. argentea levels of inclusion in the diet did not reach the threshold ratio of 210 g of CP kg-1 of DOM, implying that significant losses of ingested protein are less likely to occur with growing cattle, even at levels of offer up to 45% of the legume in the diet.











 nueva página del texto (beta)
nueva página del texto (beta)


