INTRODUCTION
Aquatic plants are interesting alternatives to conventional food sources, especially because of their accelerated growth and nutritional quality (Leterme et al. 2009). Several studies have been conducted to determine the value of different non-conventional forage resources from the nutritional point of view, both for fish and for terrestrial animals (Patra et al. 2002). Considering distribution, hydrophytes are found almost everywhere (Lowe et al. 2000). Belonging to a group classified as weeds, several species have potential forage uses, especially parrotfeather (Myriophyllum aquaticum), which is widely used as ornaments in aquariums and presents considerably rapid growth (Crow 2007). Another aquatic plant is Amazon frogbit (Limnobium laevigatum), which is typified by accelerated growth and invasiveness and is considered a pest in many countries (San Martin and Boetscher 2003). Additionally, duckweed (Lemna minor) is a small aquatic plant that must be submerged to flower and is an almost cosmopolitan species; duckweed grows quickly and efficiently, as it is able to take advantage of residual waste for its growth (Reyes et al. 2011). Finally, aquatic fern (Salvinia molesta), which has been listed as an invasive plant due to its adaptability and fast reproduction, is able to grow at high speed blocking necessary sunlight from other aquatic plants, especially the algae necessary to oxygenate water (Lowe et al. 2000). Aquaculture waste can be used as a source of nutrients for plant growth in hydroponic systems, so it is possible to incorporate hydroponics into aquaculture. In this sense, aquaponics, defined as the integration of hydroponic plant production in a recirculating aquaculture system, has been proposed as a sustainable alternative to control the accumulation of waste produced by fish farming (Rakocy 2010). In general, aquaponics is a production system in which wastes synthesized by aquatic organisms (usually fish) are converted via bacterial action into nitrates, which serve as a source of nitrogen for plants. The principle of aquaponics is based on the fact that the nutrients required for the growth and development of plant cells are very similar to the wastes that fish produce and release into the water. The fish release nitrogen directly into the water via gill excretions in the form of ammonia (NH3) (Forster and Goldstein 1969), which ionizes to form ammonium (NH4+); subsequently, by the bacterial action of the genera Nitrosomonas and Nitrobacter, the ammonium transforms into nitrite (N02-) and nitrate (N03-) (Watson 1971). Plants are part of the biological filters of aquaponic systems and take the nutrients they need, such as nitrates, from the water, thus cleaning the water that returns to the fish tank (Espinosa et al. 2016) and allowing the fish to live in a suitable environment for its growth and development, while reducing the quantity of water that needs to be replaced. Thus, the objective of the present study was to determine the biomass production of the aquaponic species Myriophyllum aquaticum, Limnobium laevigatum, Lemna minor and Salvinia molesta and their potential for feeding animals.
MATERIALS AND METHODS
Study area
The present study was carried out in the Aquaculture Laboratory (LabAc-UG) of the Veterinary and Husbandry Department at the Life Sciences Division, Campus Irapuato-Salamanca of the University of Guanajuato (20°44'34.42" N 101°19'50." W, 1745 meters above sea level).
Aquaponic experimental systems and fish
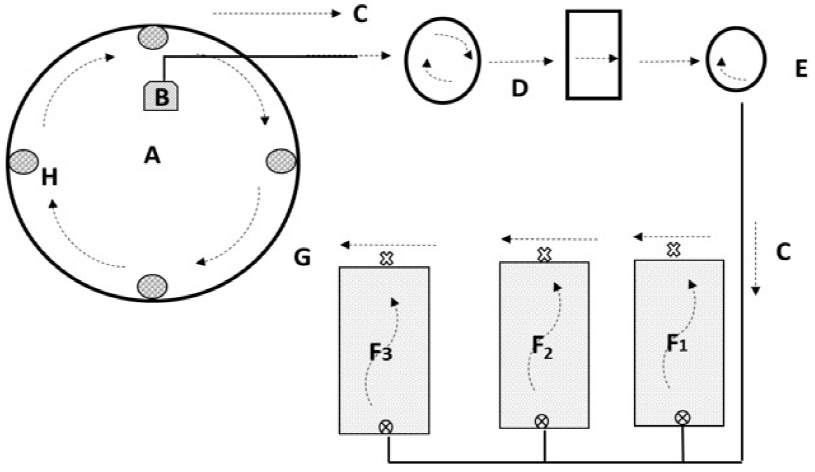
A completely randomized design with three replicates per treatment was used (one species per aquaponics system, each system with three hydroponics beds, HB). Aquaponics systems were equal and independent, each consisting of a pond (1.5 m3), a clarifier (0.25 m3), a physical filter (formed by 2 containers, each 0.06 m3, with filtering material for the retention of particles), a biological filter (a plastic container of 0.25 m3) and 3 HB (fiberglass container of 0.15 m3 with 1.0 m2 of sowing area, Vef = 0.1 m3) (Figure 1). Physicochemical parameters during the study were pH 7.8-8.2, dissolved oxygen (DO) 5-5.5 mg L-1, temperature 20-23 oC, and electrical conductivity 1000-1280 mS cm-3. The hydraulic retention time in the HB was 60 minutes, with a constant flow. PVC pipes were used for the water conduction lines, and the internal movement was carried out with a submersible pump (BOYU DJ4P-3000 ECO). The water flow was: pond → clarifier → physical filter → biological filter → HB → pond. In the pond, the air was injected (60 L min-1, BOYU ACQ-009 compressor) through a silicone hose (∅4 mm) connected to four diffusers. A total of 120 specimens of Oreochromis aureus were planted with an average weight of 6.7 g and a length of 7.2 ± 0.8 cm. The fish were fed to apparent satiation three times a day with a commercial feed (50% protein and 15% fat).
Hydrophytes
The aquatic plants parrotfeather (Myriophyllum aquaticum), Amazon frogbit (Limnobium laevigatum), duckweed (Lemna minor) and aquatic fern (Salvinia molesta) were obtained from the aquatic plant collection of LabAc-UG. A total of 200 g of vegetable biomass on a wet basis were planted in each HB. The plants were allowed to drain for 30 min in a net in the shade to remove excess water before the amount required for each species was weighed. The experiment lasted for 21 d, and at the end of the experiment, the plants were removed from each HB, the excess water was withdrawn and the biomass production per species was quantified (g m-2). Subsequently, the plant material was placed on a plastic tray (one tray per HB), and the biomass was divided into four portions per tray. Samples for the bromatological analysis were taken from each tray by sampling only one of the four portions (n = 12 per species, approx. 50-70 g). The samples were weighed and dried in an oven at 60 oC to constant weight to determine the dry matter (DM).
Chemical analysis
Three plant material subsamples per species were analyzed for organic matter (OM), crude protein (CP) (AOAC 2000), neutral detergent fiber (NDF) (method no. 6, Ankom 2014a), acid deter gent fiber (ADF) (method no. 5, Ankom, 2014b), lignin (LIG) (method no. 8, Ankom 2005), ash (method 942.05, AOAC 1995), ether extract (EE) (Randall method, Thiex et al. 2003), gross energy (GE) (IKA Calorimeter System C 2000 Basic), calcium (Ca) (AOAC 2000) and phosphorus (P) (Edmond 1969) levels.
Statistical analysis
Biomass production data were analyzed by one-way ANOVA and Tukey's test. Square root transformation of the sine-arc was applied to the chemical composition data (percentage values) (McCune et al. 2002), due to the nature of these data, and they were analyzed by the Kruskal-Wallis test (99% confidence) with the InfoStat computer program (Balzarini et al. 2008). Subsequently, matched comparisons were made between the means of the treatments to determine differences (Balzarini et al. 2008).
RESULTS
Biomass production
The biomass production of the aquatic plants grown in the aquaponics system are shown in Table 1. Significant statistical differences were observed in all variables evaluated among plant species (p < 0.05). According to our results, L. laevigatum produced the largest amount of fresh biomass matter, followed by S. molesta and L. minor; however, M. aquaticum presented the highest biomass yield of grams in dry matter (106.36 ± 2.23 g of dry matter), as well as the highest organic matter, crude protein, hemicellulose and cellulose contents in dry matter, in grams (93.73 ± 1.96, 30.38 ± 0.64, 20.48 ± 0.43, and 16.37 ± 0.34 g, respectively).
Chemical composition
The chemical compositions of the aquatic plants grown in the aquaponic system are shown in Table 2. According to the bromatological analyses, M. aquaticum contained the highest amount of DM (10.65 ± 2.13%) and OM (88.13 ± 0.13% in DM). L. minor showed the highest CP values, followed by L. laevigatum and M. aquaticum (29.90 ± 1.08, 28.96 ± 0.35 and 28.56 ± 0.11% in the DM, respectively). The highest values of NDF were recorded in S. molesta and M. aquaticum (46.21 ± 1.27 and 39.79 ± 0.30% in the DM, respectively).
Table 2 Chemical composition of hydrophytes grown in the aquaponic system.
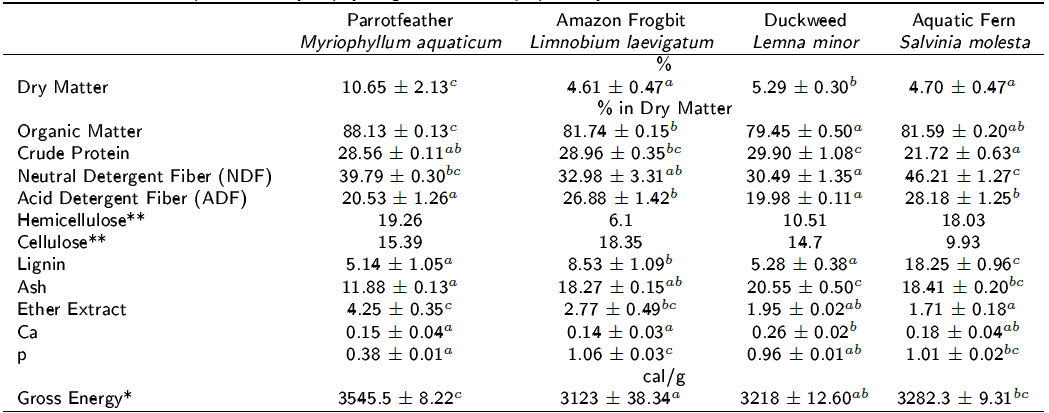
p < 0.01, Means ± SD, * Means ± SE, ** Calculated as: Hemicellulose = NDF - ADF; Cellulose = ADF - Lignin.
In addition, S. molesta had the highest value of FDA, followed by L. laevigatum (28.18 ± 1.25 and 26.88 ± 1.42% in the DM, respectively). Regarding lignin concentrations, S. molesta had the highest value (18.25 ± 0.96% in DM), and the lowest lignin concentrations were observed in M. aquaticum and L. minor (5.14 ± 1.05 and 5.28 ± 0.38% in DM, respectively). The highest ash values were recorded in L. minor, followed by S. molesta (20.55 ± 0.50 and 18.41 ± 0.20% in M, respectively). The highest values of EE and EB were observed in M. aquaticum (4.25 ± 0.35% in DM and 3.545.5 ± 8.22 cal/g, respectively). The highest concentration of Ca was recorded in L. minor, followed by S. molesta (0.26 ± 0.02 and 0.18 ± 0.04% in DM, respectively). L. laevigatum had the highest value of p, followed by S. molesta (1.06 ± 0.03 and 1.01 ± 0.02% in the DM, respectively).
DISCUSSION
To the best of our knowledge, the present study is the first to show the biomass production and chemical composition of M. aquaticum, L. laevigatum, L. minor and S. molesta grown in aquaponics and their potential for feeding animals, which opens a wide range of new alternatives for feed. The hydrophytes evaluated in the present study contained amounts of DM as those studied by Reyes et al. (2011) and Aponte et al. (2013). The hydrophytes evaluated in the present study presented similar values of lignin as those reported in M. sativa (4%, Varela et al. 2003), except for L. laevigatum and S. molesta (8.53 and 18.25%, respectively). Lignin is a component of the cell wall, so higher concentrations of lignin in the forage decrease its digestibility and the availability of energy for animals, particularly those that obtain energy from the fermentation of fiber (Hussain and Durrani 2009). Therefore, it is feasible to use L. laevigatum in animal feed, but S. molesta could present problems in relation to digestibility and consumption.
Aponte et al. (2013) obtained L. laevigatum samples from wetlands and marshes and reported a value of 16.22% PC in DM. Subsequently, these specimens were grown in a controlled environment with hydroponic nutrient solutions, analyses showed values of 4.9% DM and 2 500 cal g-1 GE, and, in dry basis, 95.1% OM, 26 to 30% PC, 1.18 to 2.55% fat, 7.63 to 8.0% crude fiber and 23.0 to 23.6% ash. According to our results, the L. laevigatum plants grown in the aquaponic system had similar values of DM, CP, and fat, higher GE values and lower ash values. Additionally, Corti and Schatteler (2002) reported that L. laevigatum under natural conditions can have as much as 16% protein in dry basis, which makes it an interesting potential forage plant; however, this concentration could change in the propagation processes under controlled conditions, where the light and space conditions are the most optimal and nutrients are not limiting, as is the case of aquaponics systems. According to Wersal and Madsen (2011), M. aquaticum has the capacity to absorb a significant amount of these elements in the water column because of its root system. Some authors who investigated Lemna sp reported a crude protein level of 29% in its DM (Ly et al. 2002), similar to the values obtained in this study. It should be noted that, in the present work, Lemna minor was the plant species with the highest amount of CP. Leterme et al. (2009) reported that Salvinia molesta contained 9.2 to 19.1% PC in its DM, a lower value than those reported here. According to Jampeetong et al. (2012), aquatic plants are good candidates for removing N in aquaponic systems and had a high nitrate uptake rate when they were supplied with only this chemical compound. Nitrate is the form of nitrogen that plants absorb and use for growth. Plants assimilate most of the absorbed nitrate in organic nitrogen compounds; the nitrate is transformed into nitrite, which is trans formed into ammonium, and the assimilated nitro gen is then incorporated into amino acids, which are used in protein synthesis (Sinha 2004), which translates into plant growth. It has been shown that the chemical composition of the nutrient solutions used in hydroponics modify the CP content in forages (Salas-Pérez et al. 2010). As part of an aquaculture recirculation system, the water in aquaponics always contains available ammonium and nitrate, which could explain the CP values observed in the hydrophytes of this study (range of 21.72 to 29.90% in DM) and those reported by Reyes et al. (2011), showing that the chemical composition of plants produced in aquaponic systems could be affected by the type of production system, which could be used to improve the plant nutritional quality. NDF values of 41.9% have been reported for Lemna minor (Ly et al., 2002), and NDF values of 51.8 to 62.9% have been reported for Salvinia molesta (Leterme et al., 2009), but the NDF percentages in the current study were lower. For ADF, the values obtained in the present study were slightly higher than the previously re ported value of 15.6% for Lemna gibba (Landesman et al., 2010) and lower than the previously reported values of 35.8 to 41.4% for Salvinia molesta (Leterme et al., 2009). For ash, values of 23% in DM for L. laevigatum (Aponte et al. 2013) and 20.1% in DM for Salvinia molesta (Leterme et al., 2009) have been reported; our results were slightly lower than those reported by other authors. According to Leterme et al. (2009), Salvinia is a good source of minerals and essential amino acids; however, its use in pig feeding is limited due to its fiber content, which results in low digestible energy and protein. The nutritional requirements of productive species vary with the physiological state, age, and sex; however, it is essential that these requirements are met by considering the quality of the ingredients produced and used in diets. In this sense, crude protein (CP) is the main nutrient in animal feed, since it fulfills various physiological functions in organisms (Elizondo-Salazar 2008). The CP requirement should be addressed in diets by considering the digestive capacity of the target species. The hydrophytes cultured in an aquaponic system evaluated in the present study met the crude protein percentages required to feed productive animals (Table 3), except for S. molesta, which did not meet the minimum protein requirement for tilapia diets. A fundamental component in the diet of herbivorous animals is the fiber present in forages. Excess fiber reduces the voluntary intake, digestibility, ruminal microbial protein synthesis, and energy in take of feed. For high-production animals that re quire significant energy inputs, fiber ranges must be established, because fiber is a factor that limits the energy content of rations. For the fiber component, NDF requirements vary. According to the results of our study, it is possible to feed these species with the evaluated hydrophytes. It is important to note that the content of DAF and lignin present in L. laevigatum and S. molesta that may limit their use as fodder, for example, increasing the content of ADF from 19-21% to 24- 26% in the diet of rabbits increased mortality during fattening (Romero et al. 2009). Variable apparent digestibility values have been observed, ranging from 15 to 60% for hemi-cellulose, 5 to 25% for cellulose and -15 to 15% for lignin (Benkeblia 2014). On the other hand, the mineral contents in ingredients and diets are of vital importance in animal feed, since suitable concentrations of minerals are beneficial for production (Table 3). Considering our results, the ash contents of the hydrophytes meet the nutritional requirements to be incorporated in food for terrestrial species. Based on the Ca g d-1 requirements for dairy cows, pigs, poultry, sheep, goats, rabbits and tilapias, the hydrophytes do not provide enough Ca element, except for the tilapia, which, according to the literature, can survive with lower levels of Ca depending on the physico-chemical characteristics of the water (Lianes 2006). However, based on the results, the aquatic plants could meet some of the requirements for P. In relation to lipids (EE), the composition of the aquaponic hydrophytes meets the minimum requirements of most species, so the use of these hydrophytes as an ingredient in an integral ration is feasible. Finally, according to our results, the aquatic plants evaluated comply with the amount of energy needed (GE) for productive species.
Table 3 Nutritional requirements for various productive animal species.
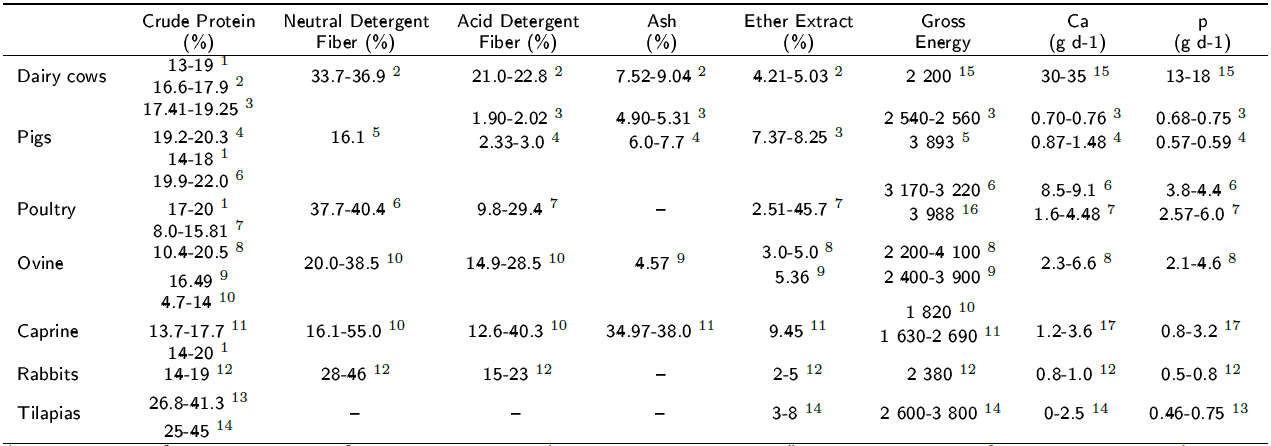
1Hou et al., 2016; 2Rossow y Aly, 2013; 3Neutzling et al., 2015; 4Sorensen y Norgaard, 2016; 5Antezana et al., 2015; 6Olkowski et al., 2016; 7Karcher et al., 2015; 8Partida de la Peña et al., 2013; 9Oliveira, 2016; 10González-Pech et al., 2015; 11Souza et al., 2014; 12Gidenne, 2014; 13Furuya, 2010; 14Lianes, 2006; 15NRC, 2001; 16Wilkinson et al., 2014; 17Elizondo-Salazar, 2008.
CONCLUSIONS
Our results show that hydrophytes cultivated in aquaponics systems can fulfill most of the nutritional quality requirements for productive animal species, so it is feasible to use them as main ingredients in whole animal rations, especially Myriophyllum aquaticum and Lemna minor, as alternatives for feeding animals. This enables a type of non-conventional fodder production, although more research is needed, particularly regarding the consumption, attractiveness, palatability and digestibility of these hydrophytes grown in aquaponic systems for different animal species. Salvinia molesta, had no value as fodder, especially because of its concentration of lignin, which could affect the digestibility and voluntary consumption.











 text new page (beta)
text new page (beta)