Introduction
The productive, reproductive and immunological capacity of the sheep throughout their postnatal life has a direct relation with the uterine environment in which they develop during the gestation. The nutritional status of pregnant ewes is the main factor related to alterations in the uterine environment, and fetal development and growth (Martin et al. 2004). In this sense, appropriate and strategic feeding before and during critical times of pregnancy ensures a correct development of the offspring in their pre- and postnatal life (Lassoued et al. 2004, Corner-Thomas et al. 2014). Maternal nutritional restriction around peri-conception has been related to alterations in the fetal growth during the last third of pregnancy (Bloomfield et al. 2004, Rumball et al. 2009), as well as post-natal problems of diseases, slow growth and low fertility in young and adult sheep (Cleal et al., 2007). Maternal peri-conceptional malnutrition has also been reported to alter fetal growth trajectory, but not birth weight (Bloomfield et al. 2013). The foregoing is due to the fact that malnutrition of the ewes during conception alters fetal programming in the early embryonic stage, which confers on the fetus physiological and metabolic adaptations that favor its survival under limited nutrient availability scenarios (Morrison et al. 2010). Although, the development of these adaptations are not completely beneficial, considering that they affect the functional competence of offsprings both in pre- and post-birth (Fleming et al. 2012).
Most reports on peri-conceptional nutritional restriction have been conducted to evaluate the long-term effects on the fetal development, since finding alterations in the fetal growth and development during the last third of pregnancy is more likely, due to the fact that around 80 % of the growth of the product occurs at this stage (Oliver et al. 2005, Morrison et al. 2010, Bloomfield et al. 2013). However, there is no evidence suggesting that the negative impact of peri-conceptional nutritional restriction can be reflected in short term, depending of the grade and time of undernutrition pre- and post-conception. In this regard, Sejian et al. (2010) noted a decrease in the vesicular development in Malpura ewes, and Muñoz et al. (2008) found lower cranial and abdominal diameter in fetuses from Greyface ewe during the beginning of the second third of gestation, both findings as result of the peri-conceptional undernutrition. Although there are also studies that reported no alterations in fetal growth at the end of the first third of gestation in Australian Merino (MacLaughlin et al. 2005) and Rahnani (Abdel-Mageed et al. 2015) ewes when they were underfed around the mating time. The number of fetuses and the period of malnutrition before and/or after conception could explain part of the inconsistencies found. In this regard, Rumball et al. (2009) found that undernutrition pre- and post-conception have different effects on fetal growth, metabolism and endocrine status during the last third of gestation. Similarly, MacLaughlin et al. (2005) and Rumball et al. (2008) found that long-term malnutrition around conception promotes different fetal growth and development, depending on the pregnancy type (single or twin). It is known that the availability of food in extensive Systems depends on the rainy season; therefore, sheep often face problems of poor nutrition. Therefore, the objective of this study was to compare different periods of undernutrition around conception, with a control in relation to maternal body status, early fetal development and birth weight of lambs in twin bearing hairsheep ewes.
Materials and methods
Animals and Treatments
The study was conducted in the autumn of 2014 within the facilities of the Sheep Experimental Unit of the Institute of Agricultural Sciences, UABC, located in the Mexicali Valley, Baja California, Mexico (32.8° NL and 115° WL). The experiment was developed using 48 multiparous Katahdin x Pelibuey ewes (live weight [LW] = 50.3 ± 0.6 kg, body condition score [BCS] = 3.0 ± 0.1 units and number of lambing = 3 to 4), which were assigned under a randomized complete block design to one of four treatments (n = 12), using LW as blocking factor. Treatments consisted in feeding the ewes with: 1) 100 % of nutritional requirements 30 days before and 50 d after mating time (control), 2) 60 % of nutritional requirements during the 30 days before mating time (RT), 3) 60 % of nutritional requirements during the 50 days after mating time (TR) and 4) 60 % of nutritional requirements during the 30 days before and 50 d after mating time (RR). Diets offered before and after the mating time were the same for all treatments and the only difference was the daily amount of dry matter (DM) offered based on the ewe LW (NRC 2007). Thus, the formulation of the diet to meet maintenance requirements in the pre-mating period was the following: metabolizable energy (ME) = 1.9 Mcal kg−1 of (DM), crude protein (CP) = 81 g kg−1 of DM, rumen degradable crude protein (RDCP)= 69 g kg−1 of DM, Ca= 2.4 g kg−1 of DM and P= 1.8 g kg−1 of DM, while for ewes in early gestation with two fetuses in the post-mating period it was as follows: ME= 1.9 Mcal kg−1 of DM, CP= 8.7 g kg−1 of DM, RDCP= 69 g kg−1 of DM, Ca= 4.1 g kg−1 of DM and P= 2.8 g kg−1 of DM. Figure 1 presents the feeding schemes for each treatment based on the amount of DM offered; given the slight variation indicated in the NRC (2007) for nutritional requirements before and after mating time in sheep, it was decided to formulate two diets, one for each experimental stage. After completing the phase in which the dietary treatments were offered, all the ewes were fed ad libitum with the post-mating diet until the day 100 of gestation, and then the diet was changed between day 101 of gestation and lambing to cover nutritional requirements for ewes with twin gestation during the last third (ME= 2.4 Mcal kg−1 of DM, CP= 11.2 g kg−1 of DM, RDCP= 86 g kg−1 of DM, Ca= 5.0 g kg−1 of DM and P= 2.9 g kg−1 of DM; NRC 2007). Table 1 shows ingredients and chemical composition of diets.
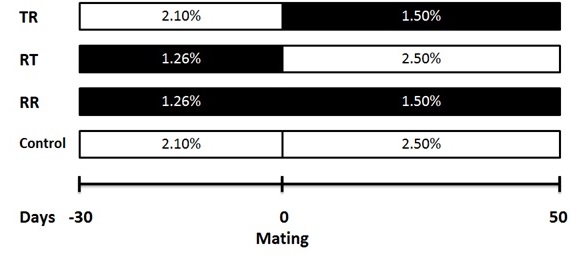
Figure 1. Feeding scheme based on the live weight percentage for ewes fed 100 % of nutritional requirements (control), or with nutritional restriction of 40 % of nutritional requirements during the pre-mating (RT), first third of gestation (TR) or in both periods (RR). The amount of dry matter offered based on live weight in control ewe was calculated according to NRC (2007).
Table 1. Ingredients and chemical composition of the experimental diets offered during the pre-mating (30 d), post-conception (first 100 days) and late gestation (last 50 d) periods.
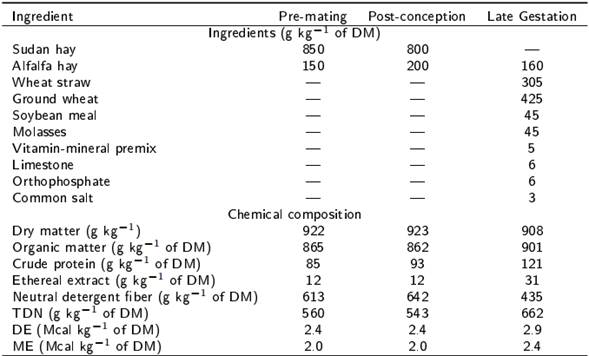
Total digestible nutrients (TDN= 91.0246 - (0.571588 x NDF), Cappelle et al. 2001), digestible energy (DE= TDN x 0.044, NRC 1985) and metabolizable energy (ME= DE x 0.82, NRC 1985).
Experimental Management
All ewes were treated with 1.0 mL of ADE Vitamins (Vidafluid®), 5.0 mL of B complex (Complejo-B®) and 1.0 mL of dewormer (Virbamec®) 15 days prior to the beginning of the experiment. In this same period, the ewes were adapted to the pre-mating diet, which contained chopped hay of Sudan grass (Sorghum x drummondii) and alfalfa (Medicago sativa L.). In the adaptation period, ewes were housed in two pens; and then, at the beginning of the experiment, they were housed in four pens of 25 m2, one per treatment. Each pen had two water troughs, galvanized sheet roof (12 m2) and enough space in the trough for all ewes to consume the diet at the same time and reduce competition. Food was offered every day in the morning (07:00 h, 60 %) and in the afternoon (17:00 h, 40 %), according to the treatments (Figure 1). The ewes were weighed individually every 10 d to adjust the amount of feed offered. The feed rejected per pen was also recorded every day to calculate the feed intake per pen. Only in the pens of control ewes (4.8 % pre-mating period and 3.7 % post-mating period from the 100 % feed offered) and TR (4.3 % pre-mating period and 0 % post-mating period from 100 % feed offered) was observed feed rejected during the study period. The water was offered freely, and the health status of ewes was checked visually every day.
Also, an estrus synchronization protocol was applied in combination with a controlled mating System to match the end of the pre-mating period and the beginning of the post-mating period in all ewes. The protocol consisted of introducing, intravaginally, sponges impregnated with 20 mg of fluorogestone acetate (Chronogest® CR) for 10 days (day 11 to 1 pre-mating) and, 24 h prior to remove the sponge, 300 IU ewe−1 of equine chorionic gonadotrophin (Folligon®) were applied i.m.. Between 12 and 36 h after the end of the protocol, ewes were exposed to white Dorper breed rams every 3 h for 30 min to detect oestrus activity. Each ewe received two matings, one at the time of estrus detection and the other 12 h later. In general, six rams of proven fertility were used; 100 % of the ewes responded to hormonal treatment and were mated.
Measurements in Ewes and Fetuses
The LW and BCS of the ewes were recorded individually at day -30, 0 and 50 of the experimental period. The LW was determined using a livestock electronic scale, while BCS was evaluated on the 5-point scale (Russell et al. 1969). With this information, LW and BCS changes were calculated in the following periods: pre-mating, first third of gestation and overall period.
At the end of the experimental period (day 50 post-mating), pregnancy diagnosis and fetal count by ultrasonography was performed. In ewes pregnant with twins, measurements of vesicle, fetus and cotyledons were performed using the methodology described by Ali et al. (2007). In vesicle and cotyledons, length, width and area were measured; while crown-rump length, fetal area, occipital-nasal length, frontal length, head area, as well as abdominal length, width and area were measured in fetuses. Images of one fetus and six cotyledons per ewe were taken to make the measurements and to ensure the care indicated by Muñoz et al. (2008), who suggest performing the evaluation of one fetus to not expose them for more than 3 min to the ultrasound waves because this affect their development. An ultrasound equipped with a 3.5/7.5 MHz multifrequential rectal transducer (LCD Ultrasound Scanner, Draminski Animal profi) was used for the measurements. The ewes were under continuous observation between days 144 and 150 of gestation to ensure that all lambs were weighed before consuming colostrum and after the mother cleaned them. Thus, lambs were identified and then birth weight and sex were recorded individually.
Statistical Analysis
Only information from the ewes pregnant with twins was used. All statistical analyzes were performed using the statistical package SAS (2004). Data collected from each variable were analyzed using the UNIVARIATE procedure to verify normality through the Shapiro-Wilk and Kolmogorov-Smirnov tests. The data of variables with absence of normality were transformed with square root to obtain normality. Subsequently, variables of LW, BCS and fetal measurements were subjected to analysis of variances under a completely randomized design using GLM procedure. Models had to treatment as fixed effect and to initial LW as covariable. In the case of lamb birth weight, the analysis was developed using the MIXED procedure and the model considered the fixed effect of treatment and nesting of breeding within the mother as a random effect. The means were compared with the Dunnett’s test, considering differences between control and each treated group at p ≤ 0.05.
Results
The control ewes presented similar (p > 0.05) LW (47.8 ± 0.8 kg) and BCS (3.0 ± 0.1 units) than ewes treated with any nutritional restriction period at the beginning of the experiment (day -30). However, RT, TR and RR ewes compared to control ewes lost (p < 0.05) LW and BCS in nutritional restriction periods (Table 2). Therefore, the control ewes had higher (p < 0.05) LW and BCS than RT and RR ewes at mating, as well as than TR and RR ewes at 50 d post-lambing (Figure 2).
Table 2. Changes in live weight and body condition score during the pre-mating, first third of gestation or both periods by effect of the peri-conceptional nutritional restriction in hair ewes.
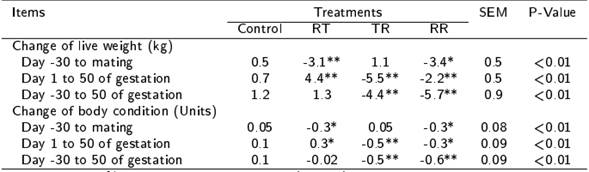
Feeding at 100 % of nutritional requirements (control), or with nutritional restriction of 40 % of nutritional requirements during the pre-mating (RT), first third of gestation (TR) or in both periods (RR). Differences between control group and any group treated with nutritional restriction are indicated with * (p < 0.05) and ** (p < 0.01).

Figure 2. Live weight and body condition score in hair ewes fed 100 % nutritional requirements (control), or with 40 % of nutritional restriction before (RT), after (TR) or before and after (RR) of mating. (* Indicate differences regarding the control at p ≤ 0.05).
Some means of vesicular and fetal measurements showed to be different between control and RT ewes (Table 3); being, in RT ewes, higher (p < 0.05) vesicular length (60.5 vs. 77.7 ± 3.2 mm) and area (20 vs. 26.8 ± 1.6 cm2), crown-rump length (54.2 vs. 60.1 ± 1.5 mm), total fetal area (6.8 vs. 9.7 ± 0.9 cm2) and abdominal area (4.2 vs. 5.2 ± 0.2 cm2). Except for occipital-nasal length, the means of vesicular and fetal measurements did not differ (p > 0.05) between control ewes and those treated as TR and RR. Fetuses from control ewes had greater (p < 0.05) occipital-nasal length than fetuses from RR ewes in 17 %. The development of placentomes in control ewes was similar (p > 0.05) to that observed in RT, TR and RR ewes.
Table 3. Vesicular, fetal and cotyledonary development by effect of the periconceptional nutritional restriction in hair ewes at day 50 post-conception.
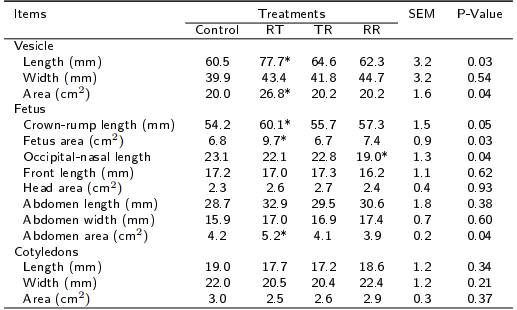
Feeding at 100 % of nutritional requirements (control), or with nutritional restriction of 40 % of nutritional requirements during the pre-mating (RT), first third of gestation (TR) or in both periods (RR). Differences between control group and any group treated with nutritional restriction are indicated with * (p < 0.05) and ** (p < 0.01).
The weights at birth were higher (p < 0.05) in lambs from RT ewes than in lambs from control ewes (2.9 vs. 3.8 ± 0.2 kg), which did not show differences (p > 0.05) with respect to lambs from TR and RR ewes (Figure 3).
Discussion
There is sufficient evidence of the negative impact of nutritional restriction on the body status of ewes during the pre-mating period (MacLaughlin et al. 2005, Grazul-Bilska et al. 2013) and first third of gestation (Cleal et al. 2007); situation that is explained by the mobilization of body reserves, as a strategy to compensate for dietary nutritional deficits (NRC 2007, Robertson et al. 2015). There are also reports indicating that ewes fed below the nutritional requirements for maintenance and production may quickly recover their normal body status when they are fed with 100 % of nutritional requirements or ad libitum with a balanced diet (Belkacemi et al. 2010). This explains, in general terms, the changes in LW and BC observed in the TR and RT ewes.
In RR ewes, it was observed that from 100 % of weight lost during the experiment, 60 % was lost in the 30 d pre-conception and only 40 % in the 50 d post-conception. This suggests that hair breed ewes have the ability to activate their adaptive mechanisms in chronic nutritional restriction scenarios, possibly of physiological and metabolic types. An immediate response from these ewes it is the mobilization of their body reserves to compensate for the nutritional deficit; however, when the period of malnutrition lengthens, they present adjustments that allow them to decrease loss of body reserves, which make a more efficient use of the available energy (Chillard et al. 1998). This study did not evaluate metabolites and metabolic hormones, but some studies indicate that ewes with chronic nutritional restriction may activate adaptive mechanisms such as: decreased levels of leptin, which motivate appetite centers to decrease energy delivery; reduction of energy use and increase in nitrogen recycling to favor the feed efficiency (Boland et al. 2001); and increase of the rate of mastication and decrease of the rate of passage to increase feed digestibility and synthesis of protein and energetic compounds by rumen microflora (Galvani et al. 2010).
Result showed that, in hair breed ewes pregnant with twins, fetal growth at 50 days of gestation was positively altered by the effect of severe nutritional restriction during the 30 days prior to mating time. Although these alterations in fetal growth were not evidenced when the nutritional restriction occurred during the first third of gestation, or it was chronic from 30 days before mating until 50 days after conception. In this regard, Jaquiery et al. (2009) mention that chronic malnutrition prior to conception may lead to low fetal growth during the gestation of single fetuses due to failures in the presence of insulin resistance, which is a normal metabolic adaptation mechanism developed by pregnant ewes to provide the greatest amount of nutrients to the fetus. For its part, Pisani et al. (2008) indicate that ewes with signs of severe malnutrition during the conception present alterations in the transcription of genes related to metabolic activity; specifically in those related with the reduction of glucose transporters and increases in PTGS2, HAS2 and leptin receptors. Therefore, the differences between our results and those reported by Jaquiery et al. (2009), it may be attributed to the number of fetuses per pregnant ewes. Consequently, it may be inferred that pre-conception undernutrition has opposite effects in ewes with simple and twin pregnancies, being favorable in the cases of twin pregnancies.
There is not precise explanation in relation to why undernutrition before conception improved early fetal growth in the studied ewes. In this regard, Underwood et al. (2005) mention that early fetal growth is positively associated with the amniotic fluid volume, which is rich in nutrients (i.e. glucose, amino acids, proteins, lipids, lactate, electrolytes, polyamines, others) and growth factors with trophic effect on muscle tissue and intestines. Also Kwon et al. (2004) found that re-feeding in ewes with chronic undernutrition after conception promoted compensatory growth in fetuses due to the increase in amino acid and polyamine concentrations in amniotic fluid, as well as in fetal and maternal plasma. Therefore, if the vesicle is full of amniotic fluid, and was larger in RT ewes, it is likely that the higher fetal growth in RT ewes is due to the effect of post-conception re-feeding after the pre-mating under-nutrition period. The re-feeding in RT ewes could favor the direction of energetic substrates, amino acids and growth factors towards the fetus, which was reflected in a greater amniotic fluid volume and early fetal growth.
In the case of TR and RR ewes, fetal and vesicular growth at day 50 of pregnancy were not altered by undernutrition periods. These results suggest possible activation of physiological and metabolic adaptive mechanisms in the mother, fetus and/or placenta, which made it possible to compensate for nutritional deficits to which the twin fetuses of these ewes were exposed (MacLaughlin et al. 2005, Oliver et al. 2005, Belkacemi et al. 2010). It should also be taken into account that twin fetuses tend to present, in a natural way, intrauterine growth retardation problems, which is an adaptive mechanism that allows them to survive with lower energy expenditure, sacrificing their growth rate (van der Linden et al. 2013). This demonstrates that twin fetuses are programmed from the stage of early embryonic development to survive in pre-natal environments with low nutrient availability. On the other hand, the size of the cotyledons was not affected by the induced nutritional restriction in hairsheep ewes during the premating period and/or first third of gestation. These results were attributed to the fact that the measurements of these structures are in line with the early development of the placenta (Osgerby et al. 2002).
The birth weights of the twin lambs were altered by the pre-mating nutritional restriction, being higher in lambs from RT ewes than in lambs from control ewes (31 %). In contrast to these results, Smith et al. (2010) reported that the birth weight was not different between twin lambs born from well-fed or nutritionally restricted ewes from 28 days before to 7 days after conception. But there are reports in ewes with singleton gestation that are in line with birth weight results of this study (Jaquiery et al. 2012). In this regard, Van der Linden et al. (2013) mention that twin fetuses may have weights above normal at the end of gestation, when placentas show high efficiency to form fetal tissue, which may be due to increased placental functionality to transport amino acids to the fetus. This could explain the higher birth weights detected in the lambs born from RT ewes. On the other hand, birth weight results in TR and RR ewes are in agreement with previous studies indicating no effect of maternal nutritional restriction before and after (Jaquiery et al. 2012, Kleemann et al. 2015) or only after conception (Cleal et al. 2007) on the weights of newborn.
Conclusions
In hair ewes, nutritional restriction in the pre-mating and/or first third of gestation causes weight and body condition losses, which are recovered quickly by offering them a balanced diet for their physiological condition. However, when the undernutrition period occurs before the mating, there are alterations in the early fetal growth and birth weight of lambs in twin bearing ewes. The nutritional restriction of 40 % of maintenance requirements during the pre-mating period followed by a well feeding with a diet formulated with 100 % nutritional requirements during gestation, improves both early fetal growth and birth weight of lambs in twin bearing ewes.











 text new page (beta)
text new page (beta)



