Introduction
Ehrlichia canis is a tick-borne obligate intracellular Gram-negative bacterium that infects canine monocytes and is the primary etiological agent of Canine Monocytic Ehrlichiosis (CME). The pathogen is transmitted to canines by the brown dog tick, Rhipicephalus sanguineus. Experimental inoculations have demonstrated an incubation period of 8-20 d in which the bacteria spread throughout the body via the mononuclear-phagocyte system (Neer and Harrus 2006). Following incubation, E. canis infection may progress into three consecutive clinical stages: acute, sub-clinical and chronic. The disease is characterized by severe clinical presentation with hemorrhages, thrombocytopenia and, in some cases, bone marrow failure (Harrus et al. 1997).
The disease is distributed worldwide, but the prevalence is highest in tropical and subtropical regions where the parasite and the vector are present. Mexico is considered an endemic region for canine ehrlichiosis caused by E. canis (Rodriguez-Vivas et al. 2000). Previous epidemiological surveys from Mexico indicated that the seroprevalence of E. canis in apparently healthy dogs was 8.7 % (Jiménez-Coello et al. 2009) and 44.1 % (Rodríguez-Vivas et al. 2005). Age ≥2 years, platelet-related bleeding and thrombocytopenia were found to be associated with higher prevalence of E. canis seropositivity. The actual prevalence of E. canis infection may differ from the seroprevalence because the presence of serum anti-E. canis antibodies indicates previous exposure to infection and not necessarily active infection (Neer and Harrus 2006). However, dogs with clinical ehrlichiosis may not have identifiable antibodies in the first days after the initial infection, before the development of a detectable antibody titer (Harrus et al. 1996, Neer and Harrus 2006).
At present, diagnosis using molecular procedures, such as nested-polymerase chain reaction (nested-PCR), is an alternative that has the advantages of higher sensitivity and specificity than serological procedures (Unver et al. 2001, Mavromastis et al. 2006, Perez et al. 2006, Aguirre et al. 2008). A large dog population (1.5 dogs per household) has been reported in the study community, representing a risk to other animals, including humans, if the disease is present (Ortega-Pacheco et al. 2007). It is important to know the prevalence of E. canis in rural communities of Yucatan, where the environment is favorable for the bacteria and the vector, in order to design and implement control measures for dogs. The objectives of this study were to estimate the prevalence of E. canis in dogs and to determine possible factors associated with its presence in a rural community in Yucatan, Mexico.
Materials and methods
Study area
The study was carried out in the rural village of Molas, Yucatan, Mexico located south of the capital city, Merida (10° 49' 01" N, 89° 37' 49" W). The climate is hot and sub-humid with summer rains. The average temperature is 26 °C, and relative humidity is 83 % with a minimum of 61 % (INEGI 2007).
Study design, study population and variables
A cross-sectional study was carried in 200 dogs between November 2009 and January 2010. Dogs were randomly selected from a population of 568 distributed in 379 houses. None of the studied animals had been included in vaccination or parasite control programs. Blood samples were collected by jugular venipuncture using 3 ml vacuum tubes containing the anticoagulant ethylenediaminetetraacetic acid, decanted within 30 min following sampling and stored at -20 °C until DNA was extracted.
All animals were clinically examined and variables such as gender, body condition, hemorrhages and presence of ticks were recorded. The age was given by the owner or calculated by dental examination, and the body condition score was assessed using a slightly modified version of the method described by Laflamme (1997).
From each sampled dog infected by ticks, at least 5 adult ticks were collected and transported to the Laboratory of Parasitology of the Autonomous University of Yucatan School of Veterinary Medicine (LP-UADY) for tick classification, following the keys of Rodriguez-Vivas and Cob-Galera (2005).
Platelet counting
Thrombocyte counts were calculated using the method described by Cheesbrough and McArtur (1979). Fewer than 200,000 platelets of blood was considered to be thrombocytopenia (Harrus et al. 1997). This procedure was carried out at the LP-UADY.
DNA extraction
DNA was extracted from 200 μl of buffy coat with a commercial kit (QIAamp® DNA Mini and Blood from QIAGEN®), according to the manufacturer´s instructions. This extraction was performed in a different room from the nested-PCR procedure.
Nested-PCR
Nested-PCR was performed to detect the 16S rRNA gene of E. canis, using the primers ECC5'-AGAACGAACGCTGGCGGCAAGCC-3' and ECB5'-CGTATTACCGCGGCTGCTGGC-3' in the first reaction and ECA5'-CAATTATTTATAGCCTCTGGCTATAGGAAA-3' and HE-3 5'-TATAGGTACCGTCATTATCTTCCCTAT-3 in the second reaction, based on previously published work (Murphy et al., 1998). The total volume of each reaction was 50 μl, with 10 μl of extracted DNA in 10 mM TrisHCI (pH 8.3), 0.2 mM dNTPs, 1.5 mM MgCl2, 50 mM KCI, 0.5 μM of each primer, and 1.25 U of Taq DNA polymerase (Qiagen). DNA of E. canis (Jake strain) purified from DH/82 cells was used for positive controls, and pure water was used for negative controls. The control used in the first reaction was also used in the second reaction. The nested-PCR was performed in a Multigene LabnetTM thermocycler. The Taq polymerase was activated for 15 min. The initial cycling consisted of 30 cycles of denaturation for 1 min at 94 °C, followed by annealing at 55 °C for 2 min and extension at 72 °C for 2 min. For the second (nested) PCR the mixture of reagents was the same, and 5 μl of the first PCR product was used as the template. The Taq polymerase was activated for 15 min. Thermocycling was in two stages: the first consisted of three cycles with denaturation for 1 min at 94 °C, followed by annealing at 55 °C for 2 min and extension at 72 °C for 1.5 min. The second stage consisted of 37 cycles, with denaturation for 1 min at 92 °C, followed by annealing at 55 °C for 2 min and extension at 72 °C for 2 min. PCR products were analyzed by 1.5 % agarose gel electrophoresis (Apex Bioresearch Products); gels were stained with ethidium bromide. All procedures were carried out at the Infectious and Parasitic Diseases Laboratory of the Autonomous University of Yucatan School of Medicine. An animal sample was considered positive when the amplicon was 389 bp in size.
Data analysis
Data were analyzed using descriptive and analytic statistics. The apparent and true prevalence and confidence interval were estimated according to the formula described by Thrusfield (2007). An initial screening of dogs infected with E. canis was performed using 2 x 2 contingency tables of exposure variables. All variables with p < 0.20 were analyzed using a logistic-binomial regression model of fixed-effects performed using SPSS v17.0 (Statistical Package for the Social Sciences 2008) software. For analysis of associated factors, animals infected with E. canis were considered dependent variables. Gender (male, female), age (<2 years, 2-5 years, >5 years), body condition (poor, medium, good), platelet-related bleeding (yes, no), thrombocytopenia (yes, no), and presence of ticks (yes, no) were considered independent variables.
Results and discussion
One-hundred and forty of the 200 dogs studied (70 %) were found to be infested with ticks. A total of 1 116 ticks (952 females and 164 males) were recovered and all were identified as R. sanguineus. This tick is a parasite of dogs that can occasionally parasitize other hosts, including humans. It is the most widespread tick in the world and is a vector of many disease agents, some of them (e.g., Coxiella burnetii, Rickettsia conorii, R. rickettsii and E. canis) being of zoonotic concern (Dantas-Torres 2010). In Yucatan, Mexico, R. sanguineus has been identified as the principal tick species in dogs (Rodríguez-Vivas et al. 2010). The apparent prevalence of E. canis was 71 % (142/200). Considering the sensitivity and specificity of the test, the true prevalence was 69.2 % with a confidence interval between 63.1 % and 74.6 %. The high prevalence of E. canis infection observed in this study using the nested-PCR method is similar to that reported by other researchers in different countries around the world using the same technique: India 50 % (Lakshmanan et al. 2007), United States of America 56 % (Kordick et al. 1999), Sudan 87.2 % (Inokuma et al. 2006). However, in Brazil, Sales et al. (2015) found lower prevalence of E. canis in dogs using a nested-PCR. Some studies in Yucatan have reported lower seroprevalences (detection of circulating antibodies) than the prevalence reported in the present study. Rodríguez-Vivas et al. (2005) reported in their work, which was carried out in owned dogs from the city of Merida and using a commercial diagnostic kit, a prevalence of 44.1 %. The prevalence in that case may have been underestimated because of the 79 % sensitivity of the test, as reported by Belanger et al. (2002), which likely produced some false negative results. Other factors that might have influenced the observed difference are sample size and bias in the selection of study animals for the serological survey. In a study performed by Jiménez-Coello et al. (2009) using indirect immunofluorescence, which is considered the reference serological test for CME, a prevalence of 8.7 % was reported. It is important to note that this study was conducted using a closed population from the city pound; this prevalence cannot be extrapolated to any other population. When estimating prevalence, the characteristics of the diagnostic test, the method of selection of the animals, and the characteristics measured must be carefully considered. In the present study, bacterial DNA from blood was measured. Despite the high sensitivity and specificity reported for this method (Aguirre et al. 2008), the prevalence is expected to be lower than that using a test that detects antibodies, which usually remain in the host animal longer than bacterial DNA.
The high E. canis prevalence in the community of Molas could also be due to environmental conditions that allow the completion of the life cycle of the vector R. sanguineus (70 % of the studied dogs were infected by R. sanguineus) and the agent E. canis, such as temperature, humidity, lack of vector control strategies and the wandering of dogs around the town. The possibility of the dogs coming into contact with other reservoirs of the disease (wildlife) is also present (Dantas-Torres 2010). The results in our study agree with those reported by Carvalho et al. (2008), who found a higher E. canis prevalence in dogs from a rural community when compared to dogs from an urban area.
The frequencies of the studied variables are shown in Table 1. 6.5 % (13/200) of the dogs presented hemorrhages and 21.0 % (42/200) had thrombocytopenia. Thrombocytopenia and hemorrhages are the main hematological abnormalities observed in dogs with CME (Dagnone et al. 2003, Rodriguez-Vivas et al. 2005). In this study a low frequency of those two variables was observed. No epidemiological associations or statistically significant differences (p < 0.05) in gender, age, body condition, platelet-related bleeding, thrombocytopenia, or presence of ticks occurrence were observed been dogs that were positive and negative for E. canis infection. These results differ from those reported by Bulla et al. (2004), Rodríguez-Vivas et al. (2005) and Santos et al. (2009), who found that seropositive and infected dogs had abnormal platelet counts with a statistically significant association between this variable and the presence of the disease. On the other hand, Santos et al. (2009) reported that 46.7 % of dogs exhibiting thrombocytopenia were not infected with E. canis, indicating the probability of another etiology. A possible explanation for the low frequency of thrombocytopenia in the dogs in the present study is that a high percentage of the infected dogs may have been in the sub-clinical stage of the disease at the time of sampling. The same could apply to the low observation of cutaneous and ocular hemorrhages, which are usually seen during the acute and chronic stages of CME (Santos et al. 2009).
Tabla 1. Variables observed in 200 dogs in a rural community in Yucatan, Mexico, included in the detection of Ehrlichia canis by PCR.
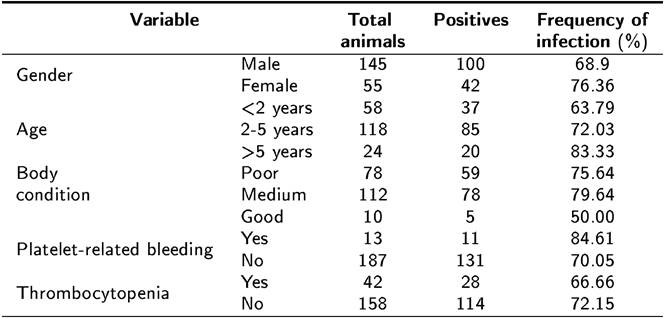
In the primary screening, age and body condition had p < 0.20; however, neither variable showed association with E. canis infection because their confidence interval included the unit when analyzed by logistic regression. In contrast to the report by Rodriguez-Vivas et al. (2005), no significant association between age and seropositivity was found. However, these results are consistent with those reported by Carvalho et al. (2008) who also found no significant association. The findings of the present study may indicate that infection of animals with E. canis can occur at any age when the animal is in contact with the vector and the bacterium.
No statistically significant association was found between gender and the presence of E. canis, which is in agreement with previous reports by Carvalho et al. (2008), Leiva et al. (2005), and Rotondano et al. (2015). Moreover, no statistically significant association was found between body condition and the presence of E. canis, indicating that this variable probably does not influence infection. Finally, it is important to point out that one of the limitations to consider when studying associations in cross-sectional epidemiological studies is that they cannot measure causality. It is not possible to determine which event occurred first, the associated factor or the disease. In conclusion, there is a high probability that dogs living in Yucatan, are infected with E. canis. It is therefore important to implement vector control measures in the community to reduce the risk of infection.











 nueva página del texto (beta)
nueva página del texto (beta)


